Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Doua K H Al homyani1, Sharifa Al Eissa2, Rushaid NA AL Jurayyan3, Nasir AM AL Jurayyan*4 and Abdulrahman A Al Bassam5
Received: November 21, 2018; Published: December 04, 2018
*Corresponding author: Nasir AM AL Jurayyan, Department of Pediatric, College of medicine, King Saud University, Saudi Arabia
DOI: 10.26717/BJSTR.2018.11.002143
Mixed gonadal dysgenesis (45X/46XY and its variants) is a form of sex chromosome DSD (disorders of sex development). The clinical presentation of such patients is highly variable and mild variants may go unnoticed. Here we discuss a 13-year-old patient with mixed gonadal dysgenesis (45X/46 XY mosaicism) who was being reared as female, presented to us with virilizing symptoms and signs. The management issues in such patients were discussed.
Keywords: Mixed Gonadal Dysgenesis; Variant; Disorder of Sex Development
Abbrevations: DSD: Disorders of Sex Development; FISH: Fluorescence in Situ Hybridization; HCG: Human Chorionic Gonadotropin; MGD: Mixed Gonadal Dysgenesis; CIS: Carcinoma in Situ
Mixed gonadal dysgenesis (45X/46XY and its variants) is a form of sex chromosome DSD (disorders of sex development). It is one of the most frequent causes of sexual ambiguity. It is a heterogeneous syndrome with a 45,X/46,XY or 46,XY karyotype, persistent müllerian duct structures, a dysgenetic testis, and a contralateral streak gonad. Functionally, the gonads were incompetent. Somatic features of turner syndrome, such as short stature, webbed neck, cubits valgus and gonadal failure may presented in these patient. Because of the presence of Y chromosome patient at risk of gonadal neoplasm, especially gonadoblastoma. So, gonadoectomy are involved. A multidisciplinary team including pediatric, endocrinologist, pediatric surgeon and psychologist should be involved. Indeed, in the ambiguous phenotypes, the decision regarding the assignment of sex must be taken as soon as possible [1-4].
A 13-year-old girl was referred to the endocrine clinic for evaluation of short stature, and signs of virilization. She was born at 36 weeks of gestation with birth weight of 2 kg and history of oligohydrominous and clitoromegally which was corrected at that time. There was no history of maternal virilization during pregnancy or prenatal exposure to androgenic drugs. There was no family history of previously affected relatives or unexplained infant death. On exam, her height was 143cm (3rd percentile), body weight was 39.9kg (25-50 percentile), BMI was21.86kg/m2. She has low posterior hair line, ptosis, shield shape chest and cubits valgus. The rest of examination was normal. Tanner stage was prepubertal External genitalia showed clitromegally, with length of 4cm and width of 2cm. Normal vaginal and urethral openings. Her karyotyping revealed 45x, 46xy + mar. SRY was positive by fluorescence in situ hybridization (FISH).
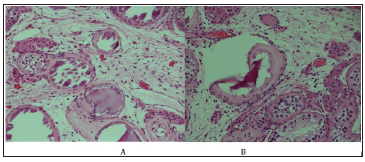
Human chorionic gonadotropin (HCG) stimulation test showed testosterone prestimulation 2.3nmol/L and post stimulation 13.43nmol/L indicating the presence of testicular tissue. LH:7.3IU/L, FSH:35.49IU/L and estradiol was less than 18.35 which go with hypergondotrophic hypogonadism. Magnetic resonance imaging MRI pelvis (Figure 1) showed: hypoplastic uterus and goA nadal dysgenesis and left gonadal tissue suggestive of testis. On pelvis laparoscopy, the uterus looked small, left gonad looked well developed while right gonad showed streak gonad and elongated clitoris, patient underwent bilateral gonadoectomy and clitroplasty. Histopathology gross description revealed left gonad ovarian like tissue attached with fallopian tube-like tissue, the ovary measure 2.0x1.0x1.0 cm, the attached fallopian like tissue measures 2.5 cm in length, no tumor was seen. Right gonad consists of one piece of fallopian tube like tissue measuring 2.0cm in length with attach tiny piece ovarian like tissue. Histological feature of left gonad consistent of infantile testicular tissue, Vas difference and collecting ducts were also seen, no intratubular germ cell neoplasia was detected (Figure 2A). Right gonad showed spermatic cord tissue with piece of vas deferens (Figure 2B). Pathologic findings were compatible to mixed gonadal digenesis.
Figure 1: MRI pelvis showing hypoplastic uterus and gonadal dysgenesiS, left gonadal tissue suggestive of testis.
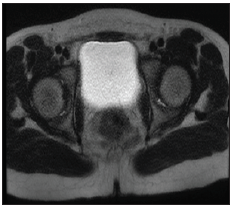
Figure 2:A: Left gonad consistent of infantile testicular tissue, Vas difference and collecting ducts were also seen, no intratubular germ cell neoplasia was detected. B: Spermatic cord tissue with a piece of vas deferencea.
This patient is genetically Turner syndrome with karyotype of 45x, 46xy + mar, who was confirmed pathologically to form mixed gonadal dysgeneis. The positive SRY gene is an evidence of Y material which was found in karyotyping. Mixed gonadal dysgenesis (MGD) comprises an heterogeneous group of diverse chromosomal, gonadal and phenotypic abnormalities which are characterized by the presence of a testis on one side and a contra- lateral streak or an absent gonad. Most patients have a 45,X/46,XY chromosomal mosaicism and germ cell tumors, such as gonadoblas- toma or dysgerminoma, which develop in about one third of patients with this syndrome [1,4]. Formation of the testis from the undifferentiated embryonic gonad depends on the presence of the short arm of the Y chromosome, containing SRY-sequences. Testosterone production stimulates development of the Wolffian system and induces male development of the external genitalia, failing which, differentiation proceeds along female lines and Müllerian structures are formed. There seems to be the necessity of a minimal amount of SRY to be present for the undifferentiated gonad to become a testis [3].
Apparently in our case, the threshold of SRY-containing cells (68%) required for the development of the embryonic gonad into a testis was obviously adequate to enable complete differentiation of the right gonad into a testis. The patients with 45, X/46, XY mosaicism are at a high risk for development of gonadal tumors. Carcinoma in situ (CIS) is thought to be a premalignant lesion leading to germ cell tumors [5,6]. Gonadoblastoma is the neoplasm most often found, and it can lead to malignant germinoma. The risk of this tumor is 15-20% and it increases with age. In the patients assigned a female gender role, the gonads should be removed, external genitalia should be repaired and oestrogen therapy should be initiated at the age of normal puberty. In patients assigned a male gender, all gonadal tissue except that which appears histologically normal and is in the scrotum should be removed, and prosthetic testes should be placed in the reconstructed scrotal sac, if appropriate. Even in the absence of evidence of testicular dysgenesis, close follow up is indicated, including a testicular biopsy at puberty and at age 20 to ascertain malignant potential [5,6] The need for androgen replacement therapy at adolescence depends on the capacity of the testes to secrete testosterone.
This is a 13-year old patient who presented with short stature, having bad virilizing symptoms, with karyotype being 45, X /46, XY + mar mosaicism. MRI pelvis showed hypoplastic uterus and gonadal dysgenesis, left gonadal tissue suggestive of testes, no intratubular germ cell neoplasia was detected in histopathology. Pathologic findings were compatible to mixed gonadal dysgeneis. Bilateral gonadoectomy and clitroplasty were done for her. Correct diagnosis and management in this patient needed a multiple disciplinary team approach (pediatric endocrinologist, pediatric surgeon, pathologist and psychologist).
The authors would like to thank Mr. Abdulrahman N Al Jurayyan for his help in preparing this manuscript and extend their thanks and appreciation to the college of medicine research Centre, Deanship of scientific research King Saud University, Riyadh, Saudi Arabia.


