Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gorachand Dutta*
Received: October 15, 2018; Published: October 29, 2018
*Corresponding author: Gorachand Dutta, Centre for Biosensors, Bioelectronics and Biodevices (C3Bio), Department of Electronic & Electrical Engineering, University of Bath, Bath BA2 7AY, UK
DOI: 10.26717/BJSTR.2018.10.001962
The main driving force of point-of-care testing require to bring the test methods conveniently and immediately to the patient. This is particularly true for the developing countries with less developed health care infrastructure. However, a simple and reliable biosensing technique to an affordable platform is often challenging [1-3]. Mostly, Enzyme-labels are used in electrochemical biosensors for signal amplification [4]. The enzyme-based biosensors are most famous because of its high and reproducible signal amplification [5]. But there is a big question mark for the stability of an enzyme and not suitable for bed-side applications. Also, another problem for the enzyme is the direct electron transfer between enzyme label and electrode is a formidable challenge because of the large electron-hoping distance between the electrode and the redox center of the enzyme label [6]. As a result, the signal amplification by enzymatic reaction is not suitable for the early stage diseases detection. Redox cycling is a process that can help to overcome this limitation by repetitively generate or consume signaling species (molecules or electrons) in the presence of reversible redox specie [7]. Many redox cycling processes can be combined with biosensor for the ultrasensitive biomarkers detection i.e. electrochemical- electrochemical (EE) redox cycling, electrochemical-chemical (EC) redox cycling, chemical-chemical (CC) redox cycling or electrochemical-chemical- chemical (ECC) [3,8].
A combination of redox cycling and electrochemical detection can play a significant role for the early stage diseases detection. Electrochemical biosensor technique is most popular and ideal technique for point-of-site application because of their low cost, high sensitivity, portable field- based size, and rapid diagnosis [9-11]. However, it is extremely challenging to originate an electrochemical point-of-site technique retaining both simplicity and very high sensitivity. There has been an increased attempt towards the development of electrochemical redox cycling techniques to develop the disposable rapid test for early stage cancer and infectious diseases biomarkers detection for point-of-care diagnosis [12-15]. Currently many biosensors using affinity binding between antigen and antibody have been developed but most of them have a drawback in terms of simplicity, rapidness, cost-effectiveness and ultra-sensitivity [16-18]. Most reported biosensors need many steps washing before the actual sensing measurement and that's why those immunoassays are not applicable for bed side application. If a wash-free electrochemical scheme is combined with the assays, this could significantly simplify the detection procedure and reduce the assay time [19-21]. In this review wash-free redox cycling technologies are focused on for simple, cost-effective and portable immunosensors that can be operated for the applications in bed-side diagnostics.
Abbreviations: EE: Electrochemical-Electrochemical; EC: Electrochemical-Chemical; CC: chemical-chemical; ECC: Electrochemical-Chemical- Chemical; EN: Electrochemical-Enzymatic; CPE: n-Conjugated Polyelectrolyte
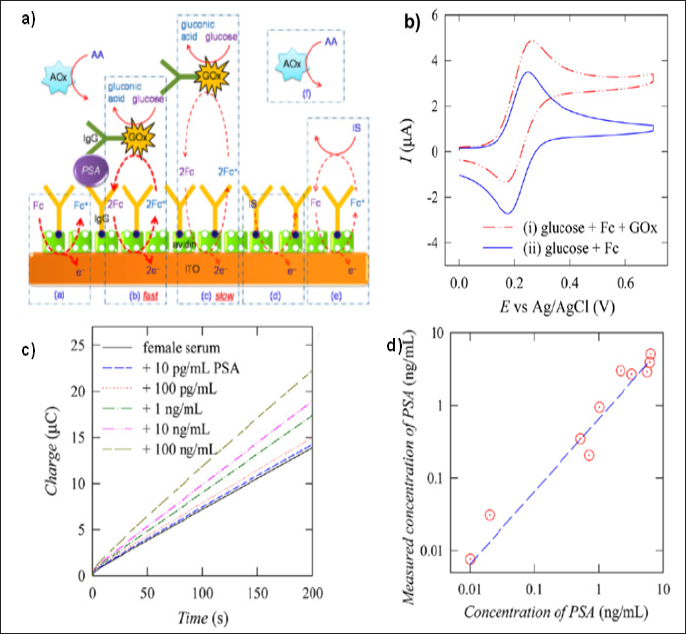
Washing-free redox cycling immunoassay technique was developed by Dutta et al. [22,23] to minimize the washing steps ofthe immunoassay that allows fast, sensitive, and single-step detection of biomarkers in serum with low interference. Electrochemical- enzymatic (EN) redox cycling (Figure1) was used to amplify the signal-to-background ratios. Biotinylated capture probe (IgG) was immobilized on the ITO electrode surface. A sample solution (contains unknown concentration of biomarker) was prepared with enzyme-conjugated IgG and enzyme-substrate, which was spiked with serum with different concentrations of target antigen. The solution mixture was then injected into the electrochemical cell and incubated for 10 min. The interference effect was minimized by applying a lower applied potential and eliminating the ascorbic acid effect. A calibration plot was obtained with increasing the target concentration. The signal was increased with the target concentration because surface concentration of bound enzyme- conjugated IgG was increased with increase in target concentration. The surface bound enzyme allowed faster electron mediation than an unbound enzyme. The limit of detection (LoD) was 1pg/mL in PBS and 10pg/mL in serum for PSA.
Figure 1: (a) Schematic diagram of a washing-free immunosensor using proximity-dependent electron mediation (b) Cyclic voltammograms for (i) PBS containing 5.0mM glucose, 100μM Fc, and 100μg/mL GOx, (ii) PBS containing 5.0mM glucose and 100μM Fc, (c) Chronocoulogram recorded at immunosensing electrodes for detecting different PSA concentrations in real samples. (d) A comparison graph between washing-free immunosensor and a commercial instrument (Reprinted with permission from Dutta et al. 2014. Copyright (2014) American Chemical Society).
An electrochemical-enzymatic redox cycling and wash-free technique was presented by Nandhakumar et al. [24] to detect cortisol where a competitive displacement method was used in human serum. The electrochemical signal was mainly contributed by the bound conjugate than the unbound one and the detection limit was ~30pM within 12min. The developed wash-free sensor can be used for simple, sensitive, and rapid point-of-care diagnosis of small molecules. An electrochemical enzymatic redox cycling- based wash-free DNA detection protocol was reported by Fang et al. [25] using proximity-dependent electron mediation. This wash-free technique could discriminate between target template DNA of Piscirickettsia salmonis and nontarget DNAs using a Zinc Finger Protein. The detection limit was approximately 300 copies in 13.2|iL, indicating an ultrasensitive detection method. An electrochemical-chemical (EC) redox cycling-based wash-free DNA sensor mediated by Conjugated Polyelectrolyte was reported by Park et al. [26] An anionic n-conjugated polyelectrolyte (CPE) label having many redox-active sites showed faster electron mediation after sandwich-type target-specific binding. The fast CPE-mediated oxidation of ammonia borane along the entire CPE backbone (EC redox cycling) affords high signal amplification and avoid the washing steps for biomarkers detection.
In this review, a simple and cost-effective wash-free redox cycling detection method was discussed for point-of-care testing such as medical diagnostics, biological research, environmental monitoring and food analysis. This simple technique can help to develop portable diagnostic biodevices which is urgently required for the developing countries with less developed health care infrastructure. In future, printing technology on flexible substrate and wash-free method could open new opportunities for the development of bioelectronics toward practical applications. Furthermore, the real sample analysis in the wash-free chip will make the diagnostic process highly applicable for bed side application.
Dr. Gorachand Dutta gratefully acknowledges to Dr. Despina Moschou for her support in University of Bath, UK and Professor Haesik Yang for his guidance during my PhD in the wash-free sensors.


