Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ulldemolins M1, Gudiol C*1,8, Pomares H2, Akova M3, Puerta Alcalde P4, Herrera F5, Martm Davila P6, Albasanz -Puig A18, Royo Cebrecos C1,8, Mercadal S2, Ayaz MC3, Cardozo C4, Torres D5, Fortun J6, Bergas A1, Laporte J1, Garcia Vidal C4, Mancho N7 and Carratala J1,8
Received: September 19, 2018; Published: October 05, 2018
*Corresponding author: Carlota Gudiol, Infectious Diseases Department, Bellvitge University Hospital, IDIBELL. Feixa Llarga SN, 08907, L'Hospitalet de Llobregat, Barcelona, Spain
DOI: 10.26717/BJSTR.2018.09.001836
Background: Current guidelines for the management of patients with febrile neutropenia don't recommend the use of empirical combination antibiotic therapy. The addition of an aminoglycoside to the recommended broad-spectrum ß-lactam could be beneficial because of the pharmacological properties of these drugs, and because it broadens the antibacterial spectrum. However, the risk-benefit of adding an aminoglycoside to the ß-lactam is far from clear, especially considering adverse events and the current situation of widespread antimicrobial resistance. We hypothesize that combination therapy may be more effective than monotherapy in this scenario; therefore, we aim to compare the effectiveness of these two strategies for the treatment of bacteraemia due to Gram-negative bacilli (GNB) in neutropenic haematological patients.
Methods: Multinational, multicentre, retrospective, observational cohort study. Adult haematological patients with neutropenia and GNB bacteraemia receiving adequate empirical ß-lactam monotherapy, or combination therapy with a ß-lactam + aminoglycoside (January 2010 - June 2017), will be analysed. The primary endpoint will be 30-day case-fatality rate. Secondary endpoints will be 7- and 14-day case-fatality rates, nephrotoxicity, persistent bacteraemia, relapse of bacteraemia, infection by resistant bacteria, and intensive care unit admission.
Discussion: Early and appropriate empirical antibiotic therapy is the cornerstone in the treatment of patients with severe infections. In patients with impaired immunity such as those with haematological diseases and neutropenia, the role of the antibiotic in the course of infection is even more crucial. Prescribing the optimal antibiotic therapy for neutropenic patients with bacteraemia due to GNB is a daily challenge for clinicians, and the impact of monotherapy with a broad-spectrum ß-lactam versus combination therapy with a broad-spectrum ß-lactam + an aminoglycoside on clinical and microbiological outcomes remains a controversial issue. A meta-analysis published in 2013, found that ß-lactam monotherapy performed better than ß-lactam-aminoglycoside combination therapy for the treatment of bacteraemia in patients with febrile neutropenia with regard to mortality, fungal super-infections, and nephrotoxicity. However, this meta-analysis was performed with data from studies performed between 1983 and 2012, when the burden of bacterial resistance was increasing but had not yet reached the levels we are facing at the moment.
Abbreviations: AKI: Acute kidney injury; ICO Hospitalet: Institut Catala d'Oncologia L'Hospitalet; IDSA: Infectious Diseases Society of America; Centro de Educacion Medica e Investigaciones Clinicas (CEMIC); ECIL: European Conference on Infections in Leukaemia; ICMJE: International Committee of Medical Journal Editors; GNB: Gram-negative bacilli; IDIBELL: Institute of Biomedical Research of Bellvitge; HSCT: Haematopoietic stem cell transplantation; ICU: Intensive care unit; EUCAST: European Society of Clinical Microbiology and Infectious Diseases; MASCC: Multinational Association of Supportive Care in Cancer; MDRGNB: Multidrug-resistant Gram-negative bacilli; REIPI: Spanish Network for Research in Infectious Diseases
Patients with haematological malignancies, and especially those with severe neutropenia, are prone to infections with high associated morbidity and mortality. Specifically, they are at high risk of Gram-negative bacteraemia due to chemotherapy-induced gastrointestinal mucositis and prolonged periods of neutropenia. Owing to the impact of infection on clinical outcomes, antibiotic therapy is usually initiated empirically upon suspicion of infection before the causative pathogen/s or their susceptibilities are identified. Historically, initial empirical antibiotic therapy for febrile neutropenia consisted in combination therapy including double ß-lactam regimens and, afterwards, aminoglycoside-ß-lactam combinations [1,2]. Pharmacological properties of aminoglycosides include fast and concentration-dependent killing of bacteria, with a post-antibiotic effect and a potential synergistic effect [3]. Moreover, addition of an aminoglycoside broadens the antibacterial spectrum, which in an era of increasing antimicrobial resistance may reduce the risk of prescribing inadequate empirical treatment and, at the same time, may provide protection against the development of bacterial resistance. However, despite these potential advantages, the use of aminoglycosides remains controversial because of their adverse events, mainly nephrotoxicity.
In this regard, clinical trials and meta-analyses have failed to demonstrate a beneficial effect of ß-lactam + aminoglycoside combination therapy over ß-lactam monotherapy on survival in patients with febrile neutropenia. In this patient population, the 2011 Infectious Diseases Society of America (IDSA) guidelines recommend the use of an antipseudomonal ß-lactam in monotherapy [4]. However, multidrug-resistant Gram-negative bacilli (MDRGNB), and particularly carbapenem-resistant strains, are spreading quickly and severely compromise patients' outcomes [5-9]. Currently, the guidelines of the European Conference on Infections in Leukaemia (ECIL) recommend applying an escalation or de-escalation strategy according to the individual risk of infection due to resistant organisms of each patient [10]. Thus, it is still a matter of debate whether monotherapy with a broad- spectrum ß-lactam may be sufficient in the empirical approach of high-risk haematological patients with febrile neutropenia in the current era of widespread antimicrobial resistance. It is also unclear whether the risk-benefit balance of combination is positive, especially regarding toxicity, considering that haematological patients are likely to be administered frequent repeated cycles of potentially nephrotoxic antibiotics and other drugs that may favour the development of kidney disease.
This study will test the hypothesis that combination therapy with a ß-lactam + an aminoglycoside may be more effective than monotherapy with a ß-lactam for the treatment of bacteraemia due to GNB in neutropenic haematological patients in an era of widespread antimicrobial resistance.
Primary endpoint: To compare the efficacy of ß-lactam monotherapy versus ß-lactam + aminoglycoside combination therapy for the treatment of bacteraemia due to GNB in neutropenic haematological patients, measured in terms of 30-day case-fatality rate.
Secondary endpoints: To compare the rates of the following events between study groups (monotherapy versus combination therapy):
a. Death at 7 and 14 days from bacteraemia onset.
b. Nephrotoxicity.
c. Persistent bacteraemia.
d. Relapse of bacteraemia.
e. Colonization/infection by bacteria resistant to study antibiotics.
f. Rate of intensive care unit admission.
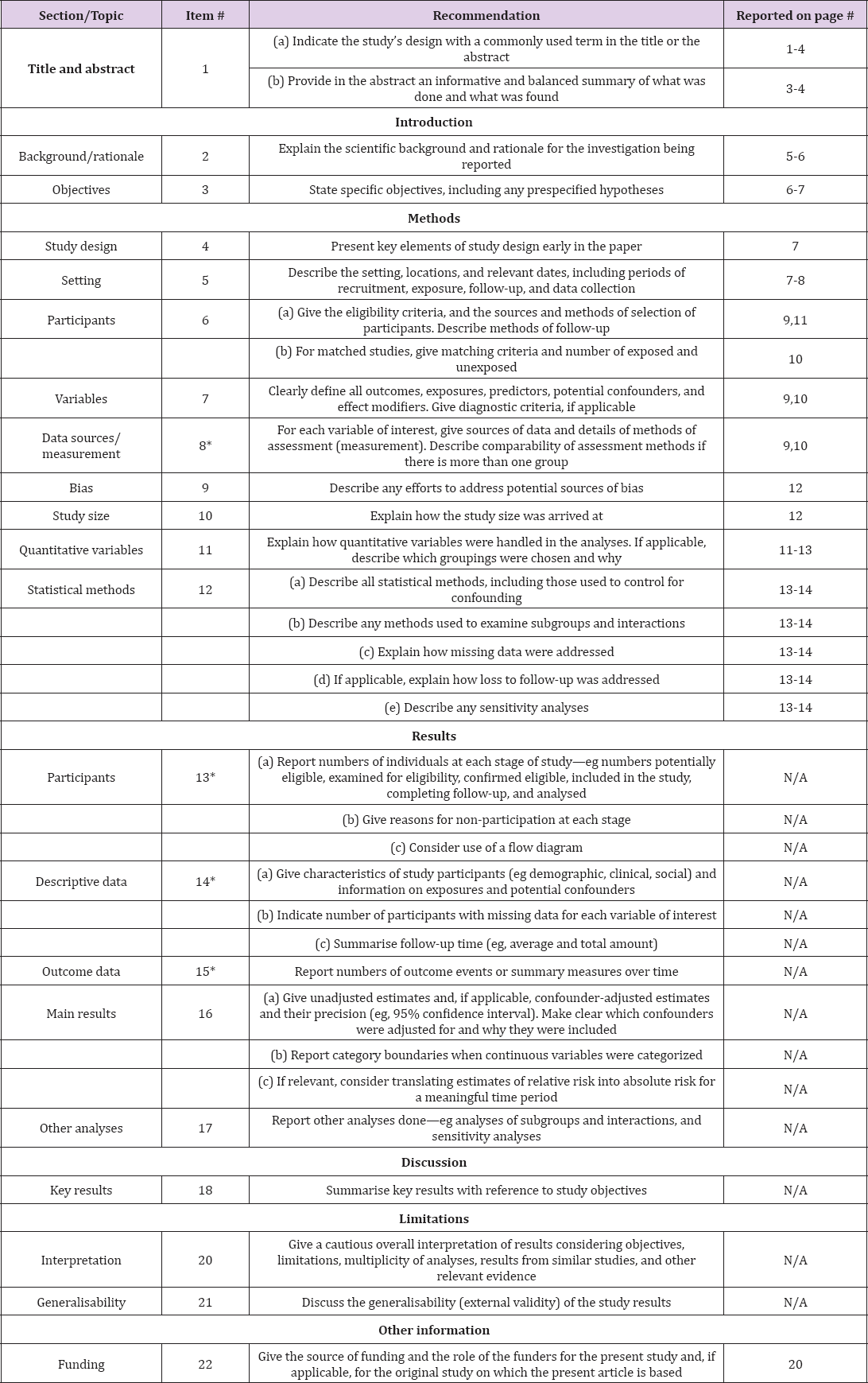
We will perform a multinational, multicentre, retrospective, observational cohort study with data collected from 1 January 2010 to 30 June 2017. This study will be conducted following the Declaration of Helsinki guidelines and in accordance with the STROBE recommendations (Table 1) [11].
Table 1: STROBE 2007 (v4) Statement-Checklist of items that should be included in reports of cohort studies.
The study will be performed at six university hospitals from three countries: Argentina (1 centre), Spain (4 centres), and Turkey (1 centre).
Data will be collected on adult haematological patients with neutropenia and at least one episode of bacteraemia due to GNB for which they received (for at least 48h) either adequate monotherapy with a ß-lactam or combination therapy with a ß-lactam + an aminoglycoside as empirical antibiotic therapy.
Patients will be identified from previous prospective databases collected by each of the participating centres. These centres will retrospectively review all episodes of GNB bacteraemia occurring in neutropenic haematological patients during the study period.
a. Adult patients (≥ 18 years).
b. Haematological disease and/or haematopoietic stem cell transplant (HSCT) recipients.
c. Neutropenia (<500 neutrophils/mm3).
d. Bacteraemia due to GNB.
e. Empirical appropriate antibiotic therapy with a ß-lactam +/- an aminoglycoside for at least 48 hours. Patients who receive at least one of the two antibiotics active in vitro against the infecting organism will be considered to have received appropriate empirical antibiotic therapy.
a. Unavailability of key data (related mainly to empirical antibiotic therapy and outcomes).
b. Receipt of inappropriate empirical antibiotic therapy. Variables
Patients' data will be collected retrospectively from prospective bacteraemia databases kept at each participating centre. The following data will be collected: sex, age, creatinine and glomerular filtration values at different times during antibiotic treatment, underlying disease and comorbidities, haematological malignancy status, blood test results including the neutrophil counts at the onset of infection, performance of haematological stem cell transplantation, risk of poor outcomes according to the Multinational Association of Supportive Care in Cancer (MASCC) index score12, source of bacteraemia, source control status, clinical and microbiological data, duration of neutropenia, prior therapies received (e.g., antibiotics, immunosuppressants), recent hospital and intensive care unit admission, recent antibiotic therapy, recent invasive therapies and procedures, prior episodes of bacteraemia, empirical and targeted antimicrobial therapy, duration of each antibiotic therapy, need for ICU admission and mechanical ventilation, persistent bacteraemia, relapse of bacteraemia, colonization and/or infection by a resistant organism, very early, early, and late case-fatality rates, and incidence and degree of nephrotoxicity
Empirical And Targeted Antibiotic Therapy: Antimicrobial therapy administered before susceptibility results become available will be considered as empirical, and antibiotic therapy prescribed according to susceptibility results will be considered as targeted.
Appropriate Antibiotic Therapy: Administration for > 48h of at least one antimicrobial agent to which the causal pathogen is susceptible according to validated laboratory antimicrobial susceptibility tests.
Very Early, Early, and Late Case-Fatality Rates: Case-fatality rates at 7, 14 and 30 days from bacteraemia onset due to any cause.
Acute Kidney Injury (AKI): AKI will be defined and classified following the AKIN criteria, in which stage 1 of AKI is defined as a rise in plasma creatinine concentration of 1.5-1.9 times baseline OR by ≥0.3 mg/dL (≥26.5 μmol/L) increase; stage 2 is defined as a rise in plasma creatinine concentration of 2.0-2.9 times baseline; and stage 3 is defined as a rise in plasma creatinine concentration of 3.0 times baseline OR initiation of renal replacement therapy and/or any use of renal replacement therapy [13].
Nephrotoxicity: AKI will be attributed to nephrotoxic drugs following the Bradford-Hill causality criteria and considering that no causes of AKI other than drugs have been identified in the clinical context.
Clinical samples will be processed at the microbiology laboratories ofeach participating centre in accordance with standard operating procedures. GNB will be identified using standard microbiological techniques at each centre. In vitro susceptibility will be determined according to EUCAST recommendations [14]. The specific mechanisms of resistance will be provided when possible according to molecular analyses. GNB will be considered to be MDR according to the definitions provided by Magiorakos et al. [15].
The follow-up period of each patient will last 30 days after bacteraemia onset.
Primary endpoint:
a) Case-fatality rate at 30 days from bacteraemia onset.
Secondary Endpoints: Except for 7- and 14-day case-fatality rates, the other outcomes will be assessed within 30 days of bacteraemia onset.
a) 7- and 14-day case fatality rate: all cause case-fatality rate at 7 and 14 days from bacteraemia onset.
b) Nephrotoxicity: Creatinine and glomerular filtration values will be collected at the start and at the end of antibiotic treatment. In patients receiving aminoglycosides, these values will also be recorded at the end of the administration of the aminoglycoside, and also after 72 hours and seven days, to assess late-onset nephrotoxicity due to these drugs.
c) Persistent Bacteraemia: positive blood cultures beyond the first 48h of appropriate antibiotic therapy.
d) Relapse of Bacteraemia: positive blood cultures due to the same GNB within 15 days of treatment discontinuation.
e) Rate of colonization/infection due to bacteria resistant to the study antibiotics.
f) Rate of intensive care admission.
This is an observational retrospective study performed with data collected prospectively on empirical antibiotic therapy of GNB bacteraemia in haematological patients with febrile neutropenia, for which the empirical antibiotic choice was at the discretion of the treating physician. Therefore, the main bias that we are facing is that the choice of monotherapy versus combination therapy may be influenced by several variables related to the patient and the clinical presentation. To account for this bias, survival analysis through Cox proportional hazards regression models will be adjusted by a propensity score for receiving combination therapy as empirical therapy [16].
The sample size will be determined by the total number of episodes of GNB bacteraemia in neutropenic haematological patients empirically treated either with te-lactam monotherapy or te-lactam + aminoglycoside combination therapy at the participating centres during the study period. Assuming 25% mortality due to GNB bacteraemia in both groups, 426 observations will be required to estimate the differences between monotherapy and combination therapy with a precision of 0.06, without loss to follow-up, with a = 0.05 and μ = 0.2 in a two-sided test.
Neutropenic patients empirically treated with combination therapy with a te-lactam + aminoglycoside will be compared with those treated with monotherapy with a te-lactam for the treatment of GNB bacteraemia. Continuous quantitative variables will be compared using the Mann-Whitney U test or t-test as appropriate. Qualitative variables will be compared using the chi-square test, and odds ratios and 95% confidence intervals will be calculated. The potential risk factors associated with mortality will be assessed by developing three multiple logistic regression models where the dependent variables will be very early, early, and late case-fatality rates. Case-fatality rates of patients treated with monotherapy or combination therapy will be compared using Kaplan-Meier curves and log-rank tests. Further, case-fatality rates at days 7, 14, and 30 will be analysed using the chi square test to compare very early, early, or late mortality between study groups.
To control for confounding variables, multivariate analysis will be performed by Cox regression, using time until death as the dependent variable and treatment with monotherapy or combination therapy as the independent variable. A propensity score for receiving combination therapy as empirical therapy will be added to the model. The propensity score (the probability of receiving combination therapy as empirical therapy) will be calculated using a non-parsimonious multiple logistic regression model in which the outcome variable will be the use of combination therapy as empirical therapy. No missing data are expected regarding the main outcomes, since the unavailability of related data is an exclusion criterion. Exploratory subgroup analyses will also be performed according to the type of p-lactam received, the targeted therapy administered, and the microorganisms causing bacteraemia (resistant or susceptible). The analysis will be performed with the stepwise logistic regression model of the R software (R v. 3.2.5).
Early and appropriate empirical antibiotic therapy is the cornerstone in the treatment of patients with severe infections. In patients with impaired immunity such as those with haematological diseases and neutropenia, the role of the antibiotic in the course of infection is even more crucial [7]. Prescribing the optimal antibiotic therapy for neutropenic patients with bacteraemia due to GNB is a daily challenge for clinicians, and the impact of monotherapy with a broad-spectrum te-lactam versus combination therapy with a broad-spectrum te-lactam + an aminoglycoside on clinical and microbiological outcomes remains a controversial issue.
As far as efficacy is concerned, the use of two antibiotics with two different spectrums of activity should theoretically provide a higher probability of covering the infecting pathogen. Furthermore, the interaction between two antibiotics with different mechanisms of action and different post-antibiotic effects may be synergistic and may improve on the bacterial kill activity of the antibiotics when administered separately [17-19].
Nevertheless, real life data on this issue are contradictory. A meta-analysis by Paul et al. including 71 trials, published in 2013, found that te-lactam monotherapy performed better than te-lactam-aminoglycoside combination therapy for the treatment of bacteraemia in patients with febrile neutropenia with regard to mortality (for monotherapy, RR 0.80, 95% CI 0.64-0.99), fungal super-infections, and nephrotoxicity (RR 0.45, 95% CI 0.35 to 0.57 for any nephrotoxicity, RR 0.31, 95% CI 0.15 to 0.63 when using a nephron-protective once-daily aminoglycoside dosing regimen). The authors concluded that monotherapy should be regarded as the standard of care for the empirical treatment of febrile neutropenic patients, and that the addition of an aminoglycoside not only failed to improve survival but was associated with significant morbidity, mainly through aminoglycoside-associated nephrotoxicity [20].
However, this meta-analysis was performed with data from studies performed between 1983 and 2012, when the burden of bacterial resistance was increasing but had not yet reached the levels we are facing at the moment [21]. Bacteraemia caused by MDRGNB is strongly associated with increased rates of treatment failure and mortality in patients with neutropenia, probably due to inappropriate empirical antibiotic therapy [22,23]. Therefore, it is not surprising that recent reports suggest that combination therapy with a ß-lactam + aminoglycoside may be associated with better outcomes in the current scenario of increasing antimicrobial resistance. In a previous study of a prospective cohort of neutropenic patients with haematological malignancies and bacteraemia, our group found that combination therapy with a ß-lactam + aminoglycoside was associated with significantly lower mortality [24]. In fact, in view of this phenomenon of increasing antimicrobial resistance, specific guidelines recommend a de-escalation approach with initial broad-spectrum antibiotics or combination therapy for patients with known prior colonization or infection with resistant pathogens, as well as in settings with high rates of antimicrobial resistance [10].
With regard to safety, the incidence of antibiotic-related adverse events (mainly nephrotoxicity due to aminoglycosides) is one of the factors that most limits the prescription of combination therapy However, it has been shown that nephrotoxicity is usually mild as long as the drug is administered using nephron-protective once- daily dosing regimens and only for short periods of time [25,26]. The short regimen is frequently used empirically in neutropenic patients until the results from the microbiology laboratory become available; once the results are known, the aminoglycoside is usually suspended in order to narrow the spectrum and avoid adverse events. The impact of short courses of aminoglycosides on outcomes has been reported by (among others) Ong and colleagues, who performed a study evaluating the effects of a short add-on course of gentamicin (maximum three days of treatment) on the rates of AKI, reversal of shock, and death, in critically ill patients admitted in the ICU with severe sepsis or septic shock [27]. The authors found that this strategy was associated with an increased incidence of AKI, but not with faster reversal of shock or improved survival. However, the study was performed in a setting with low prevalence of antimicrobial resistance and with patients who were not neutropenic.
The efficacy and safety of short add-on courses of aminoglycosides for the treatment of GNB bacteraemia in haematological patients with neutropenia in an era of increasing antimicrobial resistance is yet to be evaluated. Furthermore, therapeutic drug monitoring of trough concentrations of aminoglycosides for titrating dosing based on Bayesian methods is extensively used today in order to optimize the aminoglycoside's antimicrobial effect and, at the same time, to minimize the risk of nephrotoxicity [28].
This study aims to compare the efficacy and safety of ß-lactam monotherapy versus ß-lactam + aminoglycoside combination therapy for the treatment of bacteraemia due to GNB in neutropenic haematological patients in an era of increasing bacterial resistance. The relevance of the study lies in the fact that it targets a patient population prone to severe or even life-threatening bacteraemia due to GNB for which early empirical appropriate antibiotic therapy is likely to be the main determinant of outcomes. If we are able to demonstrate that antibiotic combination therapy with an aminoglycoside is beneficial in improving patients' outcomes, this might have an important impact on daily clinical practice.
The collection of patients’ data started on 1st July 2018, and it will be completed in 30th October 2018. Protocol version number 2.0. Date: 24rd November 2017.
The study will be conducted following the Declaration of Helsinki guidelines. On 19 October 2017, it was approved by the Institutional Review Board ("Comite Etico de Investigacion Clinica") at Bellvitge University Hospital, with reference number EPA052/17. Approval will also be sought from all relevant ethics committees. To protect personal privacy, identifying information of each patient in the electronic database will be encrypted. The processing of the patients' personal data collected in this study shall comply with the Data Protection Act 1998 and with the European Directive on the Privacy of Data. The investigator/research lead at each site will guarantee that all team members or other persons involved at his/her site will respect the confidentiality of any information concerning all study patients. The need for informed consent has been waived by the Clinical Research Ethics Committee because of the retrospective nature of the study.
The results obtained from this study will be reported at national and international conferences and in peer-reviewed journals. The main publication will be based on data from all the participating sites testing the study hypothesis against the primary outcome, and will be analysed with the support of statisticians. All presentations and publications deriving from this study will be considered as joint publications by the AMINOLACTAM study group including all the participating investigators, and they will follow the recommendations of the International Committee of Medical Journal Editors (ICMJE).
This study was supported by the Plan Nacional de I+D+i 2013-2016 and Instituto de Salud Carlos III, Subdireccion General de Redes y Centros de Investigacion Cooperativa, Ministerio de Economia, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0001) - cofinanced by European Development Regional Fund 'A way to achieve Europe", Operative Programme Intelligent Growth 2014-2020. Dr. Royo-Cebrecos received a grant from the Spanish Network for Research in Infectious Diseases to carry out the research project.1
We thank the ESGBIS and the ESGICH study groups for supporting the study. We thank CERCA Programme/Generalitat de Catalunya for the institutional support.


