Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Luisa Beltran1, Emily Leach1, Sureshni de Fonseka1, John Bolodeoku*2 and Frank Chinegwundoh3
Received: September 21, 2018; Published: October 03, 2018
*Corresponding author: ohn Bolodeoku, JB Consulting MDP Limited, 1 Bell Street, Maidenhead, Berkshire, SL6 1BU, London
DOI: 10.26717/BJSTR.2018.09.001822
Objectives: A reliable point-of-care testing (POCT) method for the quantitation of prostate-specific antigen (PSAJ may offer considerable benefits to prostate cancer patients who are undergoing expectant management in a primary care setting. The i-CHROMATM POCT method for the analysis of PSA is a novel fluorescence-based immunoassay that provides quantitative analysis of total PSA in serum, plasma or whole blood. The aim of this study was to evaluate the performance of the i-CHROMATM POCT PSA method for the analysis of serum.
Design and Methods: Serum samples (n = 54) received for PSA measurement were analysed using the routine laboratory method (Cobas® e602 Total PSA assay) and the i-CHROMATM POCT PSA method.
Results: The data showed that overall, the i-CHROMATM PSA method showed good correlation with the Cobas® PSA method (r2 = 0.9664). Results within the range of 2 - 10 μg/L showed a statistically significant mean positive bias of 7% (0.4 μg/L) on the i-CHROMATM compared to the Cobas® method, however this bias was found to be higher in the 0.1 - 2 and 10 - 100 μg/L ranges. Inter-assay precision, assessed by replicate analysis of pooled serum samples (n = 8), was 6% and 5% at concentrations of 4 and 18μg/L respectively. Performance was poorer in the lower range, with precision of 19% at a concentration of 1.8 μg/L. Other practical aspects of the method, e.g. importance of accurate reaction timing, were also assessed and found to be acceptable for use.
Conclusion: In summary, the i-CHROMATM POCT PSA method provides a reliable measurement of total PSA in serum samples within the range of 2 - 100 μg/L.
The estimation of prostate-specific antigen (PSA) levels in the blood is the most important biomarker in the diagnosis, monitoring and management of prostate cancer [1]. In the community, total PSA testing is used to monitor patients with prostate cancer and to screen men at risk of prostate cancer. Usual practice is for a blood sample to be collected at a community location, such as a GP surgery, and transported to a laboratory, or for the patient to travel to a local hospital to give the sample. The PSA result is usually communicated to the patient 48 hours later, and a further appointment is often required to discuss the results. Point-of- care (POCT) PSA testing would eliminate this delay and call-back, thus enabling a real-time discussion to take place about the PSA result and reducing the number of clinic visits required. Once the patient has made an informed decision to have the test, he could leave the setting with his PSA result and the GP would be alerted to an abnormal value the same day. This would facilitate timely discussion, further investigations, or onward referral, if necessary, to urology [2]. In a study by Wilkinson et al. [3], patients felt the ability to have an immediate discussion about the result and future management was advantageous.
In addition, Jadhav et al. [4] demonstrated that uncertainty about the future while waiting for prostate cancer test results was extremely stressful for patients. Therefore, minimising this stressful waiting period may contribute to optimising patient care. The PSA analyte is typically quantified using well-established laboratory methods. However, at present there are several POCT PSA assay systems, from different manufacturers, available on the market. The NHS Centre for Evidence-Based Purchasing (CEP) recently evaluated three quantitative methods, the Qualigen™ FastPack®, VEDALAB PSA-CHECK-1, Mediwatch PSAwatch™ and Bioscan™ systems, and one semi-quantitative method, SureScreen PSA test [5]. The evaluation found that none of these POCT PSA assays satisfy the criteria for acceptable performance. These methods did not compare favourably with assays currently used routinely in the laboratory and, with the exception of the FastPack® and the VEDALAB PSA-CHECK-1, all of the systems demonstrated poor precision. Therefore in view of the poor performance of the POCT PSA assays and the potential disagreement between laboratory and POCT PSA methods, the report concluded that it was doubtful that the introduction of a POCT PSA testing service could offer any significant improvement in the diagnosis and monitoring of prostate cancer [6].
Furthermore, none of these POCT PSA tests satisfied the acceptable performance criteria for use when testing asymptomatic men as part of the NHS Prostate Cancer Risk Management Programme [7]. There have been recent developments in quantitative POCT PSA methods such as the FREND™ PSA Plus [8], the OPKO 4Kscore® Test [9] and the i-CHROMATM PSA system [10]. The performance of some of these analysers has been evaluated in the literature in a small number of publications. One study showed that the quantitative results obtained with the OPKO 4Kscore® test using a finger stick of whole blood correlated extremely well with laboratory assays over the clinically relevant range of PSA, including at very low PSA concentrations [11]. Another paper showed that the PSAwatch™ and Bioscan™ systems demonstrated good correlation (r2 = 0.88) with laboratory results [12]. Despite the availability of a range of POCT methods for the quantitation of PSA, there have been very few publications, and there remains very little information in the public domain with regards to their performance and clinical utility. In order to address the lack of evidence base in this area, we set out to explore the possibility of incorporating the i-CHROMATM PSA method into the management of patients with prostate cancer in primary care. A key factor for this proposal is to demonstrate, through rigorous evaluation, that the i-CHROMATM PSA method meets acceptable performance criteria for this purpose. The aim of this study was to evaluate the performance of the i-CHROMATM POCT PSA method for the quantitation of PSA in serum samples.
The i-CHROMATM POCT PSA method is a quantitative assay for the measurement of total PSA in serum, plasma or whole blood using fluorescence immunoassay technology. The method uses a sandwich immuno-detection principle, such that the fluorescence- labelled detector antibody binds to the target protein in the sample. The sample is then applied onto a test strip and the fluorescence labelled antigen-antibody complex is captured by a second antibody embedded in the solid phase. The signal intensity of fluorescence of the captured complex is directly proportional to the amount of PSA present and thus enables the calculation of sample PSA concentration via a pre-programmed calibration process. The result of the test is displayed on the reader as nanograms per millilitre (ng/mL). A fluorescence-labelled control protein is included in the reaction and the intensity of the control line is measured as a quality check.
All reaction components and the meter required for performance of the assay are available from the manufacturer. The assay was performed following the manufacturer's instructions. In brief, 75μL of serum was mixed with a pre-measured volume of detection buffer containing fluorescence-labelled anti-PSA monoclonal antibodies and anti-rabbit IgG. A small volume, 75μL, of the mixture was then loaded into the sample well of the test strip and the cartridge was incubated at room temperature for 15 minutes. The intensity of the captured fluorescence-labelled PSA-antibody complexes was measured using the supplied meter, and the concentration of PSA in the sample was calculated. Assay accuracy and precision during the study was assessed using internal quality control (IQC) material supplied by the manufacturer.
Fifty-four samples used for this study were surplus, anonymised serum samples that were received by the laboratory for routine measurement of total PSA using the Cobas® e602 PSA assay. The samples selected spanned the analytical range of the i-CHROMATM PSA assay; 12 samples had PSA concentrations <2.0μg/L, 22 samples had concentrations between 2.0μg/L and 10.0μg/L, and 20 samples had concentrations between 10.0μg/L and 90μg/L.
Samples from three recent distributions of the Immunology Quality Assurance Services (IMMQAS) external quality assurance (EQA) PSA scheme (distributions 410, 411 and 412) were retrieved from storage (-20oC) and analysed using the i-CHROMATM PSA method. Scheme reports were consulted to obtain the all laboratory trimmed mean (ALTM) consensus value, which was used to investigate bias of the i-CHROMATM PSA method.
Patient serum samples were pooled to obtain three pools at concentrations of approximately 2, 4 and 20 μg/L. Intraassay precision was evaluated by analyzing each pool 8 times consecutively by a single operator. Inter-assay precision was evaluated by analyzing each pool 8 times on different days and by multiple operators. The precision of multiple readings of the same cartridge was also evaluated by measuring a single cartridge 8 times consecutively for each pool.
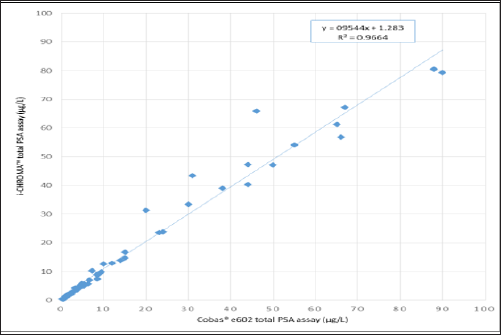
The data showed that overall, the i-CHROMATM PSA assay showed good correlation with the Cobas® e602 PSA assay (r2 = 0.9664) (Figure 1; raw data available in Appendix 1). Results within the range of 2 - 10 μg/L showed a statistically significant mean positive bias of 7% (0.4μg/L) on the i-CHROMATM compared to the Cobas® method. Results within the 0.1 - 2μg/L range showed a higher mean positive bias of 16.9% (0.1μg/L) on the i-CHROMATM compared to the Cobas® method, however this higher bias may be related to the greater degree of analytical imprecision exhibited by the i-CHROMATM in this range (see section 3.2 below). Results in the range of 10 - 100 μg/L showed a mean positive bias of 4.5% (0.8 μg/L) on the i-CHROMATM compared to the Cobas® method, however the data showed that there is a high degree of scatter around the mean of the two methods within this range, so individual results may show up to a 30% positive bias.
Figure 1: Correlation of the i-CHROMATM PSA assay and the Cobas® e602 PSA assay for the measurement of total PSA (n = 54).
Data analysis from the 6 samples from three recent distributions (410-1, 410-2, 411-1, 411-2, 412-1 and 412-2) of the IMMQAS EQA PSA scheme showed that the i-CHROMATM PSA method had a positive bias (range: 1 - 43%) compared to the ALTM consensus target value (Table 1). In addition, the results also showed that the i-CHROMATM PSA method typically had a positive bias relative to the Cobas® e602 PSA method, which was consistent with the comparison of patient samples.
Table 1: Comparison of i-CHROMATM PSA method results to EQA scheme target values and Cobas® e602 PSA method results.
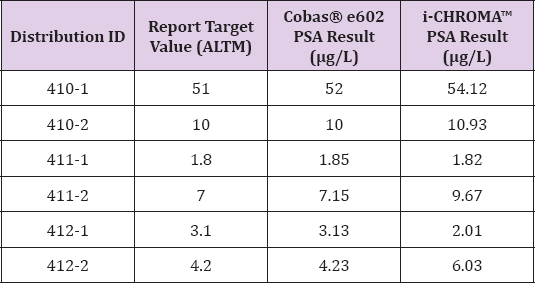
Note: ALTM=all laboratory trimmed mean; PSA=prostate- specific antigen.
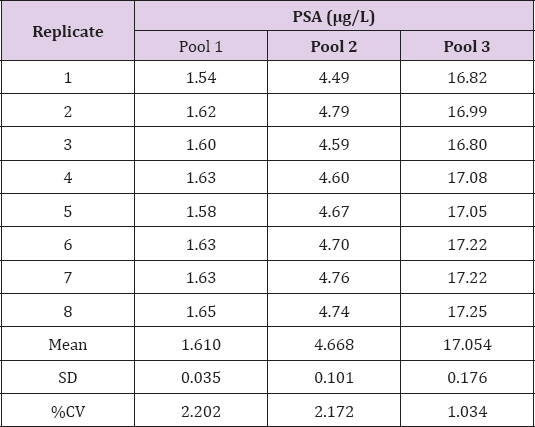
Patient serum samples were pooled to obtain three pools at concentrations of approximately 2, 4 and 20μg/L. Intraassay precision was evaluated by analyzing each pool 8 times consecutively by a single operator. The data showed that intraassay precision for the i-CHROMATM method was approximately 7% at a concentration of 4 μg/L and 4% at a concentration of 18μg/L (Table 2). Intra-assay precision was shown to be relatively poor for the lowest concentration pool, with a percentage coefficient of variation (%CV) of around 21% by a single operator (Table 2). Inter-assay precision was evaluated by analysing each pool 8 times on different days and by multiple operators. The data showed that inter-assay precision for the i-CHROMATM method is approximately 6% at a concentration of 4 μg/L and 5% at a concentration of 18μg/L (Table 3). Inter-assay precision was also shown to be relatively poor for the lowest concentration pool, with a %CV of around 19% by multiple operators (Table 3). The data showed that the reproducibility of cartridge reading was good, with %CV values of approximately 1 - 2% (Table 4).
Table 2: Intra-assay precision was evaluated by analysing each pool 8 times consecutively by a single operator.
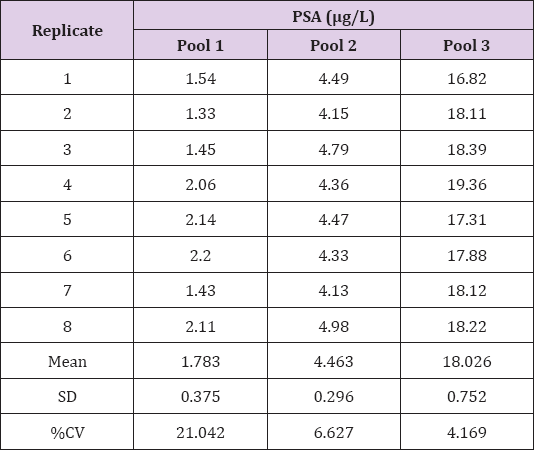
Note: PSA=prostate-specific antigen; SD=standard deviation; %CV=percentage coefficient of variation.
Table 3: Inter-assay precision was evaluated by analysing each pool 8 times on different days and by multiple operators.

Note: PSA=prostate-specific antigen; SD=standard deviation; %CV=percentage coefficient of variation.
Table 4: Precision of multiple readings was evaluated by taking 8 consecutive readings of a single cartridge.
Note: PSA=prostate-specific antigen; SD=standard deviation; %CV=percentage coefficient of variation.
The correlation of i-CHROMATM PSA results against the Cobas® e602 PSA results using patient samples showed that overall the methods compared well, however the i-CHROMATM method shows a consistent positive bias relative to the Cobas® method. Results within the range of 2 - 10 μg/L showed a statistically significant mean positive bias of 7%, which is likely to be clinically acceptable but will need to be taken into account if the i-CHROMATM PSA method is implemented. Results in the range of less than 2 μg/L showed a greater mean positive bias of 16.9%. This finding, taken together with the results of the precision studies, suggests that the accuracy and precision of the i-CHROMATM PSA method in this range is less reliable. This should be taken into account when determining the clinical utility of the i-CHROMATM system. Results in the range of greater than 10μg/L showed a mean positive bias of 4%; however, the bias for individual samples may be up to 30%. These discrepancies may reflect the heterogeneity of antigen epitopes used by the different methods and highlight the need for dual reporting for tumour marker monitoring when moving patients between methods.
It should be noted that this correlation was performed using the same sample type for both methods, however, the proposed sample type for clinical application in a primary care setting is capillary whole blood and therefore discrepancies between the methods may be greater in clinical practice. Precision studies showed that intra-assay and inter-assay imprecision were around 6% at a concentration of 4μg/L and 4 - 5% at a concentration of 18μg/L. Biological variation of PSA has been reported to be up to 18% and the desirable acceptable imprecision for PSA is 9% [13,14], therefore this level of imprecision is acceptable. This level of imprecision is also comparable to the Cobas® e602 method, which has a %CV of approximately 6% at a concentration of 3 μg/L and 4% at a concentration of 15 μg/L. However, imprecision was significantly higher at a concentration of less than 2 μg/L. Again, the less reliable performance of the i-CHROMATM method in this range should be taken into account when determining clinical utility. Intra- and inter-assay precision data were comparable, which is to be expected as the i-CHROMATM method uses independent, singleuse cartridges. It is reassuring that results appear to be consistent between multiple operators. However, it should be noted that all operators were qualified laboratory personnel, and results in a clinical setting may show a greater degree of variation. In addition, the correlation observed between the i-CHROMATM and the Cobas® e602 was comparable to correlations observed in some other PSA POCT devices previously mentioned.
Some additional practical observations were made during this evaluation that may be relevant to the use of this method in a point-of-care setting. The i-CHROMATM Reader is simple to operate, requires no regular maintenance processes, and showed no performance issues throughout the study. The sample preparation protocol is clearly outlined by the manufacturer and simple to follow. Furthermore, all reagents are supplied ready to use. However, one potential source for error is that the sample application well is not unambiguously labelled and it is possible to apply the sample directly onto the cartridge membrane by mistake. In summary, the i-CHROMATM PSA method provides a reliable measurement of total PSA in serum samples within the range of 2 - 100 μg/L. Performance in the range of 0.1 - 2 μg/L is less reliable, however may still be useful in the clinical setting as long as this limitation is taken into appropriate consideration. Further work will be required to evaluate the performance of this method for the use of whole blood samples and to compare this approach to the laboratory method that is currently in routine use. The method is relatively straightforward to use, although there are some specific features that introduce some potential sources for error. As with all point-of-care testing procedures, comprehensive operator training will be key to minimising these risks.
The authors would like to thank JB Consulting (MDP) Limited for kindly donating all i-CHROMATM reagents and use of the i-CHROMATM Reader for this study.
a. Point-of-care testing (POCT) in primary care offers considerable benefits
b. The i-CHROMATM is a novel fluorescence-based immunoassay
c. The i-CHROMATM provides quantitative analysis of prostate-specific antigen (PSA)
d. The i-CHROMATM PSA method shows good correlation with the Cobas® PSA method
e. The i-CHROMATM POCT provides a reliable measurement of total PSA in serum samples.
JB Consulting (MDP) is the UK distributor for Boditech Med Inc., the manufacturer of the i-CHROMATM system.


