Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
C Raril and JG Manjunatha*
Received: September 24, 2018; Published: September 28, 2018
*Corresponding author: JG Manjunatha, Department of Chemistry, FMKMC College, Madikeri, Mangalore University Constituent College, Karnataka, India
DOI: 10.26717/BJSTR.2018.09.001804
A simple, low cost, sensitive, a selective electrochemical method for the detection of caffeine (CA) was developed using polymethyl orange modified carbon paste electrode (PMO/CPE) by cyclic voltammetry (CV). It was found that caffeine shows well defined irreversible oxidation peak at 1350 mV Vs. Calomel electrode in 0.2 M PBS (pH 3.0) The effect of pH, Scan rate and concentration on the voltammetric response of caffeine were studied. The linear range of peak current on the concentration of caffeine in the range from 1×10-5 to 5×10-5 and 6×10-5 to 1.5×10-4 M with a low detection limit of 5.7×10-7 M and modified electrode shows a good reproducibility with a relative standard deviation of 4.16 %. The PMO/CPE electrochemical sensor showed better electrocatalytic activity compared to the bare carbon paste electrode (BCPE).
Keywords: Caffeine; Carbon paste electrode; Cyclic Voltammetry; Methyl orange
CA [1] is one of the most often used drugs. It is a naturally occurring substance found in tea leaves, kola nuts, cocoa beans, etc. with a bitter taste. It is a white crystalline alkaloid (1, 3, 7-trimethylxanthine) and it stimulates the central nervous system causing alertness. After consuming CA within minutes, the body will absorb and reaches to the brain. It also increases blood pressure, stimulating gastric secretion and increase respiration cycles. CA can cause headaches, irritability, anxiety, drowsiness, dizziness, etc. [2-4]. Some people use CA for asthma, gallbladder diseases, shortness of breath in newborns and used for weight loss [5]. CA is one of the most commonly used stimulants among the athletes. CA creams are applied to the skin to reduce redness and itching in dermatitis. Many various analytical methods have been used for the determination of CA such as High-performance liquid chromatography [6-7] Capillary chromatography [8-9], spectroscopy [10-11], electrochemical [12-13] and FTIR [14].
The developing an effective method for the detection of CA becomes an active in the field of research. Among the different methods are developed electroanalytical method having more advantageous and become the most popular method. There are few methods are reported for the electrochemical detection of CA by using modified electrodes which includes boron-doped diamond electrodes, molecularly imprinted polymer modified carbon paste electrode, carbon fiber ultramicroelectrodes, and polymer modified glassy carbon electrode. Chemically modified electrode [15-21] shows excellent sensitivity and selectivity and used for the determination of CA in beverages. In recent years carbon materials were widely used due to the broad potential window, rich surface chemistry, relatively easy electrode preparation, and low cost. The modification of the electrode surface becomes more popular in the field of electrochemistry because it can provide new and exciting properties.
In this paper, for the first time, we reported the electrochemical determination of CA by PMO/CPE by CV techniques in 0.2 M PBS having pH 3.0. The oxidation peak current of CA increased at the modified electrode suggesting the improvement of determining the sensitivity. No literature was reported on the voltammetric method for the determination of CA at PMO/CPE. The oxidation mechanism of CA was explained in Figure 1. The first step is a 2e-, 2H+ oxidation of the C-8 to N-9 bond to give the substituted uric acid, followed by an immediate 2e-, 2H+ oxidation to the 4, 5-diol analog of uric acid. The full oxidation mechanism involving overall 4e- and 4H+ [22-25].
Graphite, silicone oil and MO were purchased from nice chemical Pvt. Ltd. Cochin, India, CA was purchased from MolyChem. The phosphate buffer solution (0.2 M) was made by mixing appropriate amount 0.2 M of monosodium dihydrogen phosphate (NaH2PO4) and disodium hydrogen phosphate (Na2HPO4) and was purchased from Himedia Laboratories Pvt. Ltd. and the stock solution of MO and CA (25×10-4 M) and was prepared in distilled water. All the experiments were carried out at under room temperature (25 ± 1°C).
A three – electrode system (EA-201 Electrochemical workstation, Mumbai, India) was used for investigating the electrochemical performance of the bare and modified electrode. CV and Differential pulse voltammetry (DPV) were used for evaluating the electrochemical performance CA at bare and modified electrode in 0.2 M PBS (pH 3.0). The bare and modified electrode was used as the working electrode, a saturated calomel electrode as the reference electrode and pt. wire as counter electrode. CV experiment was conducted at the scan rate of 100 mV/s in the potential window from 800 mV to 1650 mV, and the DPV was conducted in the same potential window at the scan rate of 50 mV/s with the pulse amplitude of 20 mV. Digital pH meter, model EQ-610 was used to make the solution the having an appropriate pH for the experiment.
BCPE was prepared by mixing graphite powder 70% and silicone oil 30% in an agate mortar until to get a homogeneous mixture. Then the paste was packed in the end of a Teflon tube having 3 mm diameter. Electrical contact is provided by inserting a copper wire. The electrodeposition of PMO on to the CPE was conducted at a scan rate of 100 mV/s in the potential window from -600 mV to 1400 mV Vs. SCE for 10 cycles in 0.2 M PBS (pH 7.0) (Figure 2) and the monomer concentration was 1×10-4 M. After electrodepositing the electrode was washed with distilled water and was used for the electrochemical studies of CA.
Figure 2: Cyclic voltammogram for the electro polymerzation of 1×10-4 M MO at BCPE in 0.2 M PBS (pH 7.0) at the scan rate of 100 mV/s.
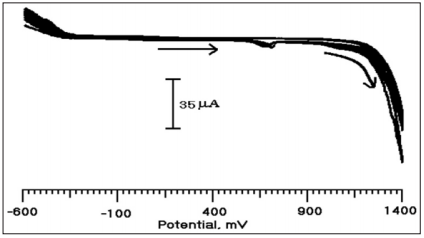
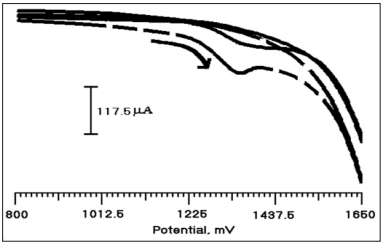
Figure 3 shows the cyclic voltammetric behavior of PMO/CPE in the presence of CA (2×10-4M) and without CA in 0.2 M PBS (pH 3.0). From the figure, it is clear that CA (2×10-4 M) undergoes oxidation at 1350 mV (dashed line) and shows that the irreversible nature of CA at the modified electrode, but in the absence of CA (solid line) there is no oxidation peak was observed. The result shows that the strong electrocatalytic effect of CA on PMO/CPE. CA is an electroactive species that undergo oxidation. Figure 4 shows the typical CVs of CA at BCPE (solid line) and PMO/CPE (dashed line). Modified electrode shows a clear peak at 1350 mV which is corresponding to the oxidation of CA. The appearance of the oxidative peak with a relatively larger peak current and improved peak potential at the PMO/CPE compared with the BCPE. The absence of a reductive peak indicated that CA undergoes irreversible oxidation at PMO/ CPE which is an agreement with previously reported work [26- 28]. Showed that the better electrocatalytic activity of CA at the modified electrode which may be due to an increased electrode surface area and improved electron exchange making suitable for CA analysis [29].
Figure 3: Cylic voltammogram of CA (2×10-4 M) with (dashed line) and without (solid line) at PMO/CPE in 0.2 M PBS (pH 3.0) at the scan rate of 100 mV/s.
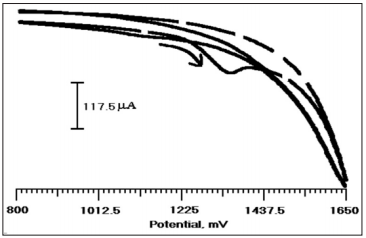
Figure 4: Cylic voltammogram of CA (2×10-4 M) with (dashed line) and without (solid line) at PMO/CPE in 0.2 M PBS (pH 3.0) at the scan rate of 100 mV/s.
The effect of scan rate was carried out to demonstrate whether the reaction is adsorption or diffusion controlled. The effect of scan rate on the anodic peak potential and peak current of CA at the PMO/CPE was examined by CV by various scan rates. Figure 5a shows the CVs of CA (2×10-4 M) at PMO/CPE in 0.2 M PBS (pH 3.0) at various scan rates. The anodic peak current increased as the scan rate increases from 100 to 300 mV/s and the peak potential towards more negative value, confirming the irreversibility of the oxidation reaction of CA at the modified electrode. Peak current and the square root of scan rate show a linear relationship with the regression equation of Ipa (µA) = 86.96+0.54 v1/2 (mVs-1)1/2 (Figure 5b) with the correlation coefficient of 0.992. Indicates that the oxidation of CA at PMO/CPE follows diffusion controlled which is agreement with reported work [30].
Figure 5: (a) Cyclic voltammogram of CA (2×10-4 M) at PMO/CPE in 0.2 M PBS at different scan rates (100, 125, 150, 175, 200, 225), (b) Ipa Vs v1/2
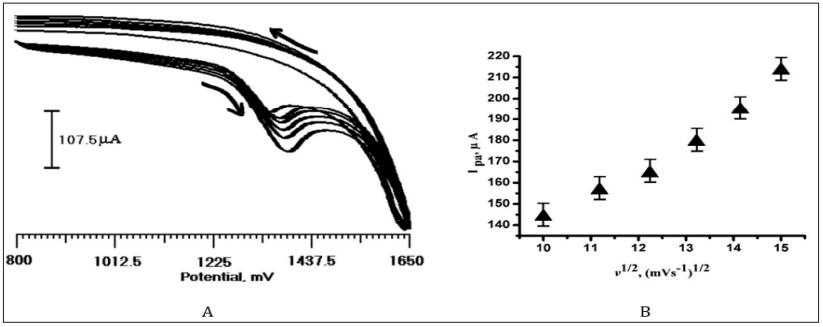
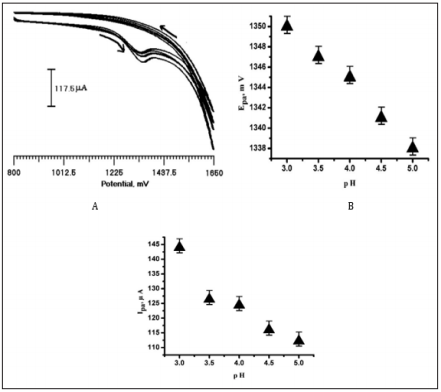
Figure 6a Shows the CV of CA at PMO/CPE in different pH (3.0 to 5.0) values in the potential window 800 mV to 1650 mV in 0.2 M PBS at the scan rate of 100 mV/s. Oxidation peak current decreased as the pH increased from 3.0 to 5.0. Shows a linear relationship between Epa Vs. pH with the regression equation of Epa (mV) = 1368.2-6 pH (Figure 6b). This decrease is attributed to the poor mass transport of CA at PMO/CPE at higher pH. The plot of pH Vs. Ipa (Figure 6c) clearly shows the effect of pH on the peak current of CA. As the pH varies the interaction of CA at PMO/CPE changes continuously. It was observed that the anodic Peak current was optimum when the solution having pH of 3.0 because of the effective interaction between PMO/CPE and CA. Thus pH 3.0 was used for the whole experiment.
Figure 6: (a) cyclic voltammogram of CA (2×10-4 M) at PMO/CPE in 0.2 M PBS at different pH values 3.0, 3.5, 4.0, 4.5, 5.0 at the scan rate of 100 mV/s, (b) Epa Vs pH, (c) Ipa Vs pH.
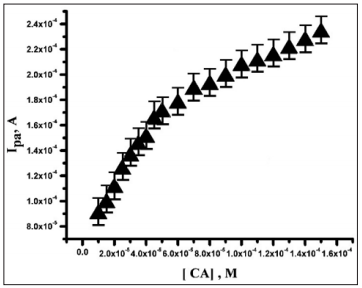
CV method was used to determine the concentration effect of CA at PMO/CPE. The plot of peak current Vs. CA concentration consisted of two linear segments 1×10-5 to 5×10-5 and 6×10-5 to 1.5×10-4M, with the regression equation of Ipa (A)=7.02×10-5 +2.0 C (M) and Ipa=1.45×10-4+0.58 C (M) (Figure 7). The decrease in the sensitivity of the second linear segment is likely due to the kinetic limitation [31]. The detection limit (LOD=3S/M; where S is the blank standard deviation for n=5 and M is the slope of calibration plot) [32-34] of CA was found to be 5.7×10-7 M. Table 1 shows the comparison of linear range and detection limit between PMO/CPE with the other electrodes. Comparison of CA sensor constructed with various electrode materials was tabulated in the Table 1 [35- 39].
Figure 7: Calibration plot for the determination of CA at PMO/CPE in 0.2 M PBS (pH 3.0) at the scan rate of 100 mV/s.
Under optimized condition, DPV was carried out in the potential from 800 to 1650 mV at the scan rate of 50 mV/s in 0.2M PBS (pH 3.0) as shown in Figure 8. The oxidation peak of MO at BCPE (at 1381mV) and PMOMCPE (at 1328 mV). An excellent enhancement of oxidation peak current was observed at PMOMCPE (2.7 times higher than BCPE). Shows the best electrocatalytic effect at PMOMCPE.
The stability and reproducibility of PMO/CPE were the measures to estimate the sensing performance of the developed electrode. RSD of Ipa was obtained 4.16% which indicates that the developed electrode is having acceptable reproducibility for the obtained result. This also shows that the no fouling of the electrode surface. The operational stability of the PMO/CPE remained 99% of initial current with continues running even after 20 cycles. This indicates that the electrode having good stability.
This work demonstrates the ability of PMO/CPE for the electrochemical determination of CA. The best response of the sensor was achieved in 0.2 M PBS pH 3.0. The oxidation peak current of CA was proportional to the concentration range from 1×10-5 to 5×10-5, and 6×10-5 to 1.5×10-4 M with the low detection limit of 5.7×10-7 M. Moreover, modified electrode shows excellent stability and reproducibility.
We gratefully acknowledge the financial support (KFIST) from the VGST, Bangalore under Research Project. No. KSTePS / VGSTKFIST (L1) 2016-2017/GRD- 559/2017-18/126/333, 21/11/2017.


