Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Chu Thi Thao1#, Do Thi Hoai Thu1,2#, Bui Viet Anh1, Truong Linh Huyen1, Nguyen Van Phong1, Nguyen Thanh Liem1 and Hoang Thi My Nhung1,2*
Received: September 03, 2018; Published: September 10, 2018;
*Corresponding author: Hoang Thi My Nhung, Vinmec Research Institute of Stem cells and Gene Technology, VNU University of Science, Hanoi, Vietnam
DOI: 10.26717/BJSTR.2018.08.001711
Introduction: Natural killer (NK) cells are central components of the innate immunity. They have ability to kill a wide range of cancer cells and are a promising tool for both autologous and allogeneic immune enhancement therapy in cancer treatment. Actually, NK cells can be derived from multiple sources such as: peripheral blood, cord blood... Among these, cord blood (CB) is known as an ideal for NK cells expansion because it occupies a higher percentage of NK cells compare to the peripheral blood and is a rich source of hematopoietic stem cell as well as progenitor immune cells. The objectives of this study were to invent a reasonable approach for expanding a relevant number of NK cells from human cord blood to clinical application. Methods: At the initial step, cord blood was collected from the patients and then the mononuclear cells were obtained by density centrifugation with Ficoll.
After that, these cells were cultured in 2 stages: stimulation and expansion with inactivated autologous plasma and BINKIT expansion kit contains several kinds of growth factors which are specified for NK cells. The immunophenotype of NK cells was analyzed every 2 days through %CD56+CD3- by Flow cytometry technique. The ex-vivo activation and expansion of NK cells was performed in GMP grade -clean room for about 3 weeks. Results: After culturing periods, the average NK cell count post-expansion was 2040.6 ± 1463.5 x10A6 and the average cell fold expansion was 814.4 ± 560.9, the expanded cells presented 90.6% ± 8.9% purify of CD56+CD3-, the percentage of CD56 bright CD3- cells was 84.2% ± 14.4%. Conclusion: Human cord blood has a significant potential to expand NK cells to relevant number for clinical use and our method are suitable for getting a relevant number and quality of NK cells to the clinical use.
Abbreviations: NK: Natural Killer; UCB: Umbilical Cord Blood; CTL: Cytotoxic T Cells; IFN-y: Interferon Gamma; IL: Interleukin; ILCs: Innate Lymphoid Cells; KIRs: Killer Cell Immunoglobulin (Ig) like Receptors; MHC: Major Histocompatibility Complex; MNC: Mononuclear Cell; PBMCs: Peripheral Blood Mononuclear Cells
Nowadays, cancer is still a leading cause of death in the world. However, the standard treatments for cancer such as surgical therapy, radiotherapy and chemotherapy usually bring multiple side-effects to patients. In some recent years, the invention of cell therapy has opened brighter future in some life-threatening diseases treatment, especially cancer. This approach helps patients to avoid immunodeficiency through sufficient provision of immune cells as well as stimulating these cells to kill specifically cancer cells. Clinical trials of cell therapy for treating many different cancers are currently ongoing. In fact, there are many types of immune cells which are used for cell therapy such as: NK cells, cytotoxic T cells (CTL), dendritic cells (DC), y5T cells and a^T cells [1]. Natural Killer (NK) cells are a subtype of type 1 ILCs (innate lymphoid cells), which play significant roles in innate immune responses.
NK cells can produce cytokine and have cytotoxicity to kill both viral infected and tumour cells. Because of the ability to secrete immunostimulatory cytokines like IFN-y, NK cells can manipulate not only tumour growth but also metastasis [2]. Therefore, NK cells have been known as promising tools for cell therapies in cancer treatment. Initial clinical trials have demonstrated that the infusion of NK cells is entirely possible and safe with no side effects and minimal toxicity to patients [3]. NK cells can be determined by the expression of CD56 and the absence of the T cell marker CD3 on their surface. Besides, most human blood NK cells also express CD16, which is involved in recognition of antibody-coated cells [4]. NK cells can be divided into two main types: CD56bright and CD56dim populations [5,6]. The CD56bright cells have low or absent expression of CD16 and killer-cell immunoglobulin-like receptors (KIR), whereas the CD56dim cells express both CD16 and KIR [7,6].
The function of NK cells is regulated through the interaction between activating receptors and inhibitory receptors [4]. The lack of combination between inhibitory KIR receptor on NK cells surface and MHC-I on target cell surface lead to the stimulation of activating receptors, and then helps NK cells to kill target cells. Therefore, the KIR mismatch assists NK cells in expressing more killing activity [8]. This is also the reason why adoptive cell transfer (ACT) approaches using allogeneic NK cells have been more effective for cancer immunotherapy [9]. In order to conduct the clinical application about NK cell-based immunotherapy, it is needs to obtain a sufficient number of NK cells with high cytotoxicity The sources of NK cells include umbilical cord blood (UCB), bone marrow, peripheral blood (PB) and embryonic stem cells. Actually, UCB not only has higher proportion of NK cells but is also an easy collecting source.
Furthermore, the establishment of UCB bank in the world now assists UCB being preserved for a long time. This leads to UCB can be used as a ready source for NK cell-based therapy. Nevertheless, although UCB contains greater frequencies of NK cells as opposed to PB, the numbers of obtained NK cells are still really small because of the limited UCB volume. This is a major hindrance in providing adequate numbers of NK cells for clinical trial [10]. The main objectives of this study were to optimize the procedure of expansion of NK cells from human cord blood in order to obtain a larger number of NK cells and higher purify of CD56+CD3- in population. The next stage is activation of these NK cells to increase the capacity of killing cancer cells effectively.
Human cord blood which is collected directly from the umbilical cord of the new born baby at Vinmec International Hospital. Before collection, the mother was diagnosed healthy and does not carry any of the following viruses: HIV, HBV, HPV, HCV. All the pregnant woman singed the written consent which is approved by the Ethics Committee of Vinmec International Hospital.
The whole cord blood was firstly centrifuged at 1700xg for 10 minutes. After centrifugation, whole blood is separated into 2 layers: plasma layer above and blood cell layer below. Then, the plasma layer was collected by pipetting. This plasma was then heat inactivated at 58oC for 60 minutes. After inactivating, plasma continued to be centrifuged at 1700xg for 5 minutes to collect the supernatant plasma and discard the pellet at the bottom of the tube.
Cord blood after plasma collection is diluted with PBS, followed by dropping down slightly to Ficoll-Paque/Lymphoprep (Stem Cell Technologies, CA) with volume proportion 2:1, and then the mixture was density centrifuged at 840xg for 20 minutes. The Ficoll solution plays a role as a tool to separate the whole blood into many layers follow the density gradient. After centrifugation, the mixture was divided into 4 layers, from top to bottom: plasma, a layer of mononuclear cells (MNC) called buffy coat, Ficoll, and erythrocytes. The MNC were collected and then washed with PBS 2 times.
The stimulation culture process was implemented within 3 days (day 0 to day 3). UCB-MNCs were cultured in initial NK medium and initial NK cocktail (Biotherapy Institute Japan) containing IL- 2, OK432, zoledronic acid and 10% heat-inactivated autologous plasma in an initial NK flask (Biotherapy Institute Japan) for initial activation. This flask was immobilized with anti-CD3 monoclonal antibody and anti-CD16 monoclonal antibody. The cell density at seeding was 1 x 106 cells/ml.
After 3 days of stimulating cultivation, the cells need to be cultured in new condition which is free from the anti-CD3 antibody, anti-CD16 antibody, OK432, and zoledronic acid or the like. These factors were removed by centrifuging the medium contains cells which has undergone the stimulation step at 270 xg for 8 minutes and removing the supernatant. Then, the cells were transferred to an anti-CD3 antibody and anti-CD16 antibody-uncoated flask and cultured in subculture medium (Biotherapy Institute Japan) supplemented with IL-2 and 10% heat-inactivated human plasma. Subculture medium was added depending to the cell number every 2 days. The cells were incubated at 37 °C with 5% CO2.
Cells were stained with these following antibodies: Anti-CD3- Pacific Blue, Anti-CD4- APC-Alexa Flour 750, Anti-CD8- FITC, Anti- CD56-PE, Anti IgG1- Pacific Blue, Anti IgG- APC- Alexa Flour 750, Anti IgG1- FITC, Anti IgG1- PE (Miltenyi Biotec, Germany). Stained cells were analysed using Navios flow cytometer with Navios software (Beckman Coulter, CA).
Data was statistically analysed by Microsoft Excel version 2013.
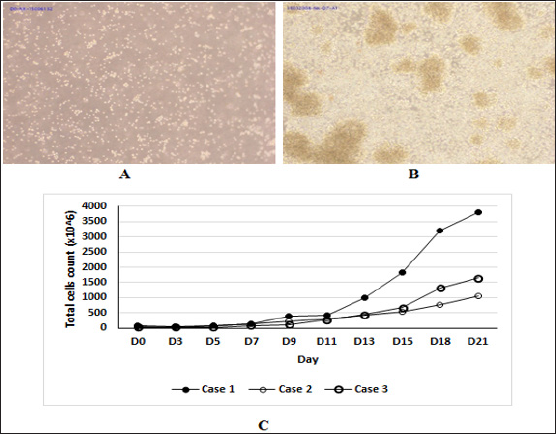
The cells at day 0 distributed uniformly in population (Figure IA). From day 3, there was the appearance of cell clumps (Figure IB). Cell population reached the log phase from D11 of culture (Fig. IC). NK cells were not only dominant during cell culture period but also significantly increased the number. The average final total cell counts we obtained from three samples was 2185.3 x 10A6 (ranging from 1102 - 1651.9 x 10A6) and the average cell fold expansion was 42.3 ± 18.3. Notably, the average NK cell counts post-expansion we obtained was 2040.6 x 10A6 (ranging from 871.7 - 3681.9 x 10A6) and the average cell fold expansion was 814.4 ± 560.9 (Table 1).The viability of cells at the day of ending culture was 98.6% ± 0.6%.
Figure 1: The morphologies of the NK cells and the number of total cells in case 1. (A) The NK cell population at day 0 (Objective 10x). (B) The NK cell population at day 7. There were clumps in cell population (Black arrows) (Objective 10x). (C) The time course of growth cells from three samples for 21 days.
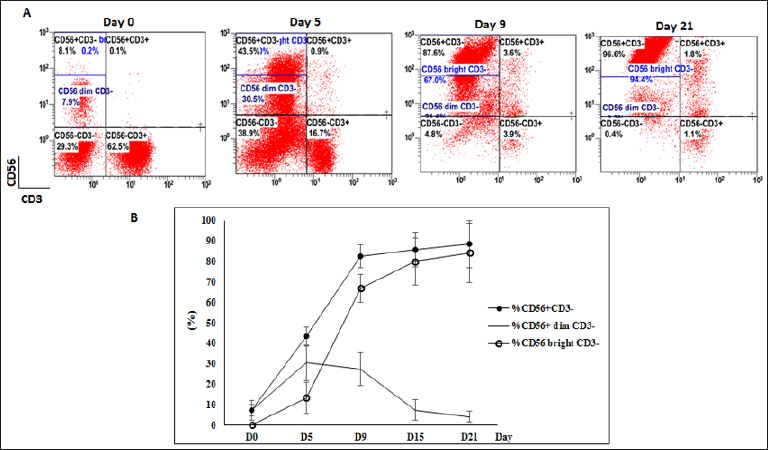
In average, the expanded NK cells (CD56+CD3-) presented 90.6% ± 8.9% purify of immune cell population (ranging from 80.7% to 96.8%). After 21 days of expansion culture, the percentage of NK cell increased by 17.8 ± 9.3 fold. There was a significant change in markers expression during culturing process (Figure 2A). The percentages of CD56dim CD3- cells at day 0 was 7.4 ± 3 %. At the initial day, the CD56-CD3+ population was dominant, until day 3 the CD56+CD3- population started rising sharply and reached >43% at day 5. From day 3 to day 10, the rate of NK cells increased dramatically and rapidly. From day 10, the rate of NK cell continued to slowly increase and peaked at 88.5 ± 11.5 %.
There were a gradually rise in the percentage of CD56bright CD3- NK cells. In average, the percentage of CD56bright CD3- increased from 0.2% ± 0.1% at the initial day and peaked at 84.2% ± 14.4% at the final culturing day. Simultaneously, we also observed that an increase in CD56 expression went parallel with a rise in the percentage of CD56 bright CD3- NK cells (Figure 2B). The more CD56 expression, a more number of CD56bright CD3- NK cells appeared in population. At the final day, the expression of CD56 was strongest led to the rate of CD56bright CD3- NK cells reached highest point at 94.4% (case 1) and 74 % (case 2). In average, the percentage of CD56bright CD3- increased from 0.2% ± 0.1% at the initial day and peaked at 84.2% ± 14.4% at the final culturing day.
Figure 2: The phenotype of immune cell population. (A) The flow analysis of case 1 at day 0, day 5, day 9 and day 21. (B) The correlation between the proportion of total NK cells CD56+CD3- and CD56dim CD3- and CD56bright CD3.
The NK expansion kit BINKIT which we used for culturing includes initial flask, initial medium and initial cocktail. The initial flask is coated with anti-CD3 antibody and anti-CD16 antibody. These antibodies may bind to the surface receptors of cells other than NK cells (CD3, CD16) and then stimulating these cells to release many liquid growth factors which are needed for NK expansion. IL-2 is known as the most optimal for NK proliferation. Actually, IL-2 enhanced at least 10-fold more NK cell expansion compared to IL- 4, IL-7, or IL-12 [11]. The initial cocktail contains IL-2 (Interleukin -2) which joins in signal pathways to proliferate NK cells and form the NK function. Concretely, these factors enhance the cytotoxicity of NK cells by stimulating the secretion of IFN- y which is needed for killing other cells (cancer cells or infected cells) [12]. The initial medium contains OK-432, this serve as an immune adjuvant capable of activating, for example, monocytes, through the binding to the surface TLR of the monocytes so that immune response is activated [13].
Another important component is zoledronic acid - a kind of bisphosphonate buffer. This acid inhibits the intracellular synthesis of Farnesyl Pyrophosphate (FPP), resulting in the accumulation of its precursor Isopentenyl Pyrophosphate (IPP). As a result, the immune response of the organism can reportedly be activated [13-15]. There are many different factors which are essential for stimulation culture as well as expansion culture process. In 2011, Jan Sphanholtz and his research group also reported their results about NK differentiation from CD34+ UCB cells. The CD34+ UCB- derived cells were expanded for 2 weeks. From day 14, the cells were cultured in 2 ways. In the first way, they used the culture bag for CD34+ expansion and combine with medium containing SCF, IL-7, IL-15 and IL-2 within 6 weeks. As the result, the mean total cell expansion was 1300 fold, the NK cells product was 9001900 x 10A6 and %CD56+CD3- was 71%±9% [16]. Their process prolonged 6 weeks as opposed to our 3 week-process, which can lead to the cell exhausted as we observed at the end of the culture (3 weeks) the dominant of crooked cells. In the second way, they used a bioreactor system for cell culturing for 6 weeks and obtained much better results: the mean total cell expansion was 2100 fold, high pure NK product with 92%±2% and total NK cells were 1600 - 3700 x 10A6 [16].
As in Shah and his colleagues' report which was published in October 2013, the proportion of CD56+CD3- obtained was > 95% and a 2389-mean fold expansion of NK cells derived from frozen UCB was achieved after 14 day. These results are much higher in comparison with our results. The main cause of this may be the fact that their method for UCB NK cells expansion was different from our approach. They use artificial antigen-presenting cell (aAPC) and cytokines including IL-2, IL-15 and FLT-3 ligand. The aAPC led to the generation of expanded UCB NK cells that displayed increased expression of NK cell activating receptors, increased perforin and granzyme expression [17]. These results are really impressive because although the time of culturing process is shorter, the number of NK cells and the rate of purify NK cells are both much higher than our results. This demonstrates that although there were some growth supplements which are the same as ours, the culturing conditions in Anushruti's procedure are more specific and effective for NK expansion. Similarly, Vasu S et al also expanded cord-blood NK cells using irradiated Epstein-Barr virus- transformed lymphoblastoid feeder cell lines from only 1 ml of umbilical cord unit. At day 21, the number of NK cells was 430 x 106 (range 44 - 4321 x 106) [18]. However, this method involved feeder layer, this led to the certain stage in which interaction between NK cells and strange cells. Therefore, it was difficult to control the influence of these feeder cells to NK cells. This is also a drawback of this method in comparison with our approach.
CD56bright have higher frequency in UCB as opposed to peripheral blood while CD56dim plays a reverse trend. In fact, upon stimulation with cytokines such as IL-2 or IL-12, not only the number but also the cytotoxic activity of CD56bright NK cell subsets dramatically increase. The percentage of NK cells in population was determined exactly by %CD56+CD3-. Moreover, in 2004, Ferlazzo reported that culturing in IL-2 can induces not only the proliferation but also the efficient cytotoxic activity of CD56bright. Therefore, we focus on the change in %CD56 bright to evaluate the increase of activated NK cells. Meanwhile, our culturing condition is the combination of IL-2 and some other growth factors such as antibodies. This contributed in the activation of NK cell. At initial day, the rate of CD56dim NK cells was dominant in population. From day 5 to the final day, the rate of CD56dim NK cells declined dramatically. Conversely, the proportion of CD56 bright rose significantly to > 90%. This means that the function of NK cells had been modified comparable with initial stage. This also demonstrates that the growth supplements in our culturing condition were efficient in activating NK cells and orienting the function expression of CD56 bright NK cells.
Human cord blood has a significant potential to expand NK cells to relevant number for clinical use. We were success to expand NK cells from Vietnamese human cord-blood with significantly pure (more than 90%), high viability (more than 98%) and the number of NK cell were relevant to clinical application (2040.6 x 10A6, ranging from 871.7 to 3681.9 x 10A6). These NK cell products are suitable for clinical trials in order to apply in cancer treatment.
The author(s) declare that they have no competing interests
This study was funded by Vingroup JSC (grant number DT-01)


