Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mehdi Ebrahimi*
Received: September 05, 2018; Published: September 07, 2018;
*Corresponding author: M Ebrahimi, 34 hospital road, Sai Ying Pun, Prince Philip Dental Hospital, The University of Hong Kong, Hong Kong
DOI: 10.26717/BJSTR.2018.08.001707
Currently, there are large numbers of commercial biomaterials that are available for therapeutic and regenerative purposes. A general overview of these biomaterials reveals that they are not officially certified for application in patients with religious concern. For example, the majority of these biomaterials are of animal origin (i.e., porcine) or have been prepared using animal derivatives. From the religious aspect, use of these biomaterials is strictly prohibited in some faiths such as Islam and Hinduism, however, this issue is largely neglected. Simultaneously, the patients are becoming more educated demanding the assurance that use of health products do not counteract with their faiths. This paper highlights the possible impact of the patient faith on biomaterials application with respect to the informed consent and medical ethics, indicating the need for new strategies to fill this gap.
Nowadays, hundreds of biomedical and pharmaceutical products are available for therapeutic applications in orthopedic, trauma, maxillofacial, and dental fields (Figure 1). Selection and application of these biomaterials are influenced by different factors such as the purpose of treatment, the biomaterials properties, and the patient factors, i.e., economy, age, and religion [1]. There are religions such as Islam and Hinduism that restrict their followers to a certain type of products or prohibit them from others. According to the Islamic concept the products used, applied or ingested by followers should be lawful (Halal), wholesome, pure, clean and nourishing (Tayyeb). As such the non-Halal sources which are strictly prohibited includes; alcoholic drinks and intoxicating drugs, pork and its by-products, products of dead animals, blood, products of animals not slaughtered according to Islamic requirements, and products from human cadaver [2]. Similarly, Hindu adhere to Sattva concept where they avoid any kind of animal derivatives having solely vegetarian or lacto-vegetarian lifestyle (Sattvic diet) [3]. With a quick look at the market, one can find a large number of commercialized non-Halal or non-sattvic biomaterials that are in daily use worldwide but not authorized to be used in patients with religious concern. This highlights an important question regarding the criteria of their applications in medical practice and the level of clinicians and patients awareness. Furthermore, it involves the issue of informed consent in the medical law and ethics where the patient has the right to be involved in the clinical decision regarding their treatment. As such, they should be completely informed about the nature of the applied or prescribed biomaterials [4,5].Unfortunately, this issue has not been given a priority and it is frequently neglected by many medical and dental practitioners, in addition to the unawareness of the majority of patients. Therefore, there is an urgent need to educate both the professionals and the patients in order to respect the medical ethics and avoid any legal consequences in medical practice.
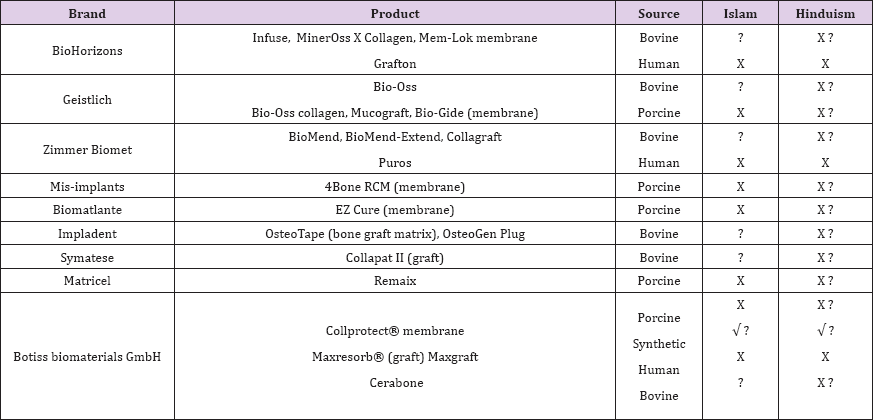
In general, the biomaterials are classified into the natural and synthetic types. The natural biomaterials could be derived from different sources including human cadaver, cow, fish, pig, etc. The synthetic biomaterials are commonly produced in the laboratory (Table 1). The natural and synthetic biomaterials are commonly applied in the medical and dental fields. Examples of such applications in medical practice are cosmetic surgery (Aquamid®), bulking agent (Bulkamid®), ophthalmic applications (Etafilcon A®), surgical dressings (Amerigel®), osmotic laxatives (MiraLAX®), and, controlled release drugs (paclitaxel) [6,7]. In addition, in dental surgical practice, one of the most common grafting procedures is bone grafting surgery to repair the bony defects [8,9] (Table 2). This surgical procedure can be performed using any of four available options:
Table 2: Examples of commercial biomaterials applied in dental practice. (x) not permissible, (?) questionable, (V) acceptable.
1) autograft that is taken from the same individual,
2) allograft that is taken from human cadaver,
3) xenograft that is taken from animals such as bovine, porcine, or equine bone, and,
4) alloplasts that are synthetic.
Biomaterials derived from the sea (i.e., fish, shellfish), alginate, chitosan, silk, and alloplast are generally considered permissible in Islamic and somehow in Hinduism faiths. However, alloplastic biomaterials seem to be the perfect choice in patients with religious restrictions as these biomaterials are solely produced in the lab during chemical processes. Nevertheless, the religious concerns of Halal and Sattva have invited serious debates in various aspects due to advancement in processing technologies. As such, it is important to clarify that the non-Halal and non-Sattvic issues can also extend to the trace additives (i.e., preservatives, binders, or plasticizers) that could be utilized during processing of biomaterials. For example, there could be ingredients of GMO (Genetically Modified Organism) within the production process and undisclosed non-Halal/non- sattvic ingredients that counteract with religious concerns [10].
Therefore, an additional effort is required to understand the nature and detailed composition of available biomaterials. This could be a challenging task but remains the entire responsibility of the clinician to be familiar with the nature of the applied biomaterials and secondly to inform and consent the patient. Although Good Manufacturing Practices (GMP) by FDA, WHO, and other standard agencies control the safety and quality of products, they do not look into the product from the religious aspect. To fill this gap, the manufacturing companies, research centers, and related organizations and agencies are responsible for the introduction of new policies for marketing and application of different biomaterials from the religious aspect.
This paper highlights the importance of the patients' faith in the application of biomaterials. This issue should be taken into consideration during treatment planning and before application of biomaterial in the patient with the religious concern, in particular, those of Islamic and Hinduism faiths. The impact of this issue should be evaluated from the perspective of medical ethics and patient informed consent and medical right. As such, the universities, the related governmental sectors, and in this context, the Islamic/Hinduism organizations are responsible for setting the required criteria and policies regarding marketing and applications of biomedical materials in patients with religious restrictions.


