Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Eman Sayed Mohammed1*, Asmaa M El Kady2, Asmaa Gahlan Youseef3 and Amal A Hassan4
Received: August 28, 2018; Published: September 04, 2018
*Corresponding author: Eman Sayed Mohammed Lecturer of Parasitology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
DOI: 10.26717/BJSTR.2018.08.001688
Background: Trypanosoma lewisi is an extracellular hemoflagellate protozon of rats. T lewisi was not considered a pathogen for human, However, cases of human trypanosomiasis caused by T. lewisi have been reported
Objective: There is little data on the prevalence of T. lewisi in Rattus norvegicus rats in Egypt, so this study aimed to determine the prevalence of T. Lewisi in Rattus norvegicus in Abu Rawash, Giza, Egypt from November 2016 to October 2017.
Materials and Methods: 117 rats were trapped around human population in Abo Rawash, Giza, Egypt. Thin and thick blood films were prepared and examined microscopically. Experimental infections of 8 white rats were done for confirmation of infection and assessment of parasitaemia.
Results: 19 (15.8%) out of 117 Rattus norvegicus were found to be infected with T lewisi. Experimental infection of 8 white rats confirmed the infection with Trypanosoma lewisi and showed boost in parasitaemia till the 9th day then the level of trypanosomes in blood was constant.
Conclusion: Trypanosoma lewisi is prevalent in examined Rattus norvegicus which reverse that the theory of high immunity of Rattus norvegicus will prevent infection with Trypanosma lewisi.
Keywords: Rodent; Trypanosoma Lewisi; Prevalence; Rattus Norvegicus; Egypt
Rodents are largest group of important animals, because they can survive in the harsh environmental condition and in several locations and live at the expense of humans, invade their dwelling, contaminated food materials, and subsequently transmit diseases to them [1] as they are reservoir hosts for a large number of ecto- and endo- parasites with great zoonotic importance [2] Trypanosoma lewisi is a specific hemoprotozoa of the rat (Rattus rattus and Rattus norvegicus), it is transmitted via faces of Xenopsylla cheopis, Nosopsyllus fasciatus, Ctenocephalides canis and C. Felis [3-5]. Furthermore, the infection of rat with T. lewisi raise its susceptibility to other micro-organisms such as Salmonella typhimurium [6] and Toxoplasma gondii [7] which represent a great public health and Rattus norvegicus are good transmitters of them [8]. On other hand, several rodent species were found in Egypt Governorates as Rattus norvegicus, R. rattus frugivorous, R. r. alexandrinus, Mus musculus and Acomys cahirinus. The flea species attacking rodents were Xenopsylla cheopis and Leptopsylla segnis. R.norvegicus was the highest manifested one with fleas because they survive where the favorable conditions for fleas breeding are available [9] The present study aimed to screen Rattus norvegicus reared around human communities at Giza, Egypt for T. lewisi infection.
The present study was conducted in Abu Rawash, Giza, Cairo.117 Rattus norvegicus was trapped around the human population, from November 2016 to October 2017. All the rats were trapped alive using steel wire traps with baits. Trapped rats were killed humanely by placing the trapped rat into a bag containing cotton wool soaked with chloroform. species for all rats captured were determined based on descriptions by [10-12] Blood was collected from the heart and thin and thick blood smear was prepared using Giemsa stain and examined under oil immersion at 1000x magnification for identification of parasite. Olympus compound blood microscope (Olympus Corporation, Japan) was used for photomicrography and images were captured using Olympus CK41 Digital Camera (Olympus Corporation, Japan). Experimental infection of 8 white rats were done to confirm the infection and to assess parasitaemia which were infected experimentally by intraperitoneal injection of blood samples positive for T lewisi (obtained from positive Rattus norvegicus examined in the present study), blood samples were obtained of experimentally infected rats every three days and examined microscopically for the presence of infection and follow up of parasitaemia. Rats were sacrificed four weeks post-infection.
19 (15.8%) out of 117 wild rats (Rattus norvegicus) weighing between 175 to 260 g were found positive for trypanosome lewisi through microscopic examination of Giemsa stained peripheral blood films. Characteristic morphological features of Extracellular hemoflagellate are well marked in Figure 1. Total length of trypomastigotes, including flagellum was 31-44 μm. Trypanosoma lewisi parasites were identified based on using an immersion objective as described by [4] and T. lewisi as the species has already been documented to cause infection in wild rats and other rodents. Moreover, experimental infection of 8 white rats results in 100% of them. Infection was confirmed by the presence of T. lewisi in peripheral blood films as shown in Figure 2 where the multiplying trypanosomes appear in blood smear. Parasitaemia in experimentally infected rats showed steady raise till the 9th day post infection then parasite level in blood was constant till rats scarification.
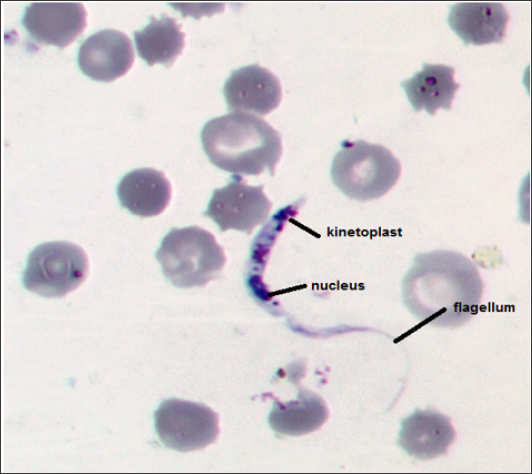
Figure 1: T. lewisi in Rattus norvegicus blood smears (100 x) with a very sharp posterior end, a subterminal kinetoplast, oval nucleus toward the anterior end and a well-developed flagellum.
Many studies have been conducted in various parts of the world to determine the prevalence of parasitic infections in murine population. In the present study, the prevalence of T. lewisi observed was 15.8% which is in accordance prevalence recorded by [13] which was 13.2%. On the other hand [9] founded that Trypanosoma lewisi prevalence in Rattus rattus. spp was very high (51.2%) with no infection in Rattus norvegicus in some rural areas in Abu Alnomros Center, Giza. The overall prevalence of rats found to be infected with T. lewisi in the present study (15.8%) is higher than that for several studies in different countries which have recorded values of 11.8% in Colombia [14] 4.6% in New Zealand (Laird 1951), 8.9% in Nigeria [15] 11.4% in Hawaii [16] and 13.2% in the USA [17]. But it is lower than that observed in India (82.30%), Venezuela (21.30), Brazil (27.7%), Italy (20%) and in Malaysia (25.2%) by [18-22] respectively.
These variation is might due to difference in geographical location, sample size and presence of vector Parasitaemia in experimentally infected rats showed steady increase of blood level of the trypomastigotes till the 9th day post infection then parasite level in blood was constant till rats scarification which is well known about T. lewisi as after a period of rapid multiplication of trypanosomes (10 days), they stop growing and their numbers stabilize for several weeks, then the parasites disappear from the blood so the solid immunity of rat might develop against T. lewisi infection. Furthermore, the infection by T. lewisi in rats produces immunosuppresion that increases the susceptibility of these animals to infection by Salmonella typhimurium and T. gondii, the importance of this observation lies in that Rattus spp in a determined area might have a greater probability of the appearance of outbreaks of salmenollosis and toxoplasmosis disease [7] The elimination of rats as one of control measure is much recommended to overcome disease associated with them
In the present work we described the presence of T. lewisi in Rattus norvegicus in Giza, Egypt to determine the prevalence of common zoonotic parasitic infections in rats.


