Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Davide Schiffer1, Marta Mellai2, Cristiano Corona3, Cristina Casalone3* and Laura Annovazzi
Received: August 21, 2018; Published: September 04, 2018
*Corresponding author: Cristina Casalone VD, Head of the Neuropathology Laboratory, Istituto Zooprofilattico del Piemonte, Liguria e Valle d'Aosta, Via Bologna 148, 10154 Turin, Italy
DOI: 10.26717/BJSTR.2018.08.001680
The problem of the location of glioblastoma (GB) stem cells (GSCs) and of their relationship with the microenvironment is discussed. Also in our experience, they are located in perivascular and perinecrotic niches. In the latter, as an alternative hypothesis to their origin by hypoxia through Hypoxia Inducible Factor 1, they could represent the remnants of stem cells/progenitors that populate the highly proliferative areas of GB where necrosis develops in avascular zones due to the imbalance between the high proliferation rate of tumor cells and the low one of endothelial cells. On the whole, tumor stem cells and tumor non-stem cells are in equilibrium between differentiation and stemness, regulated by the microenvironment with the possibility of an interconversion of the cell statuses.
Abbreviations: CSCs: Cancer Stem Cells; NSCs: Neural Stem Cells; GB: Glioblastoma; GSCs: Glioblastoma Stem Cells; OPCs: Oligodendroglial Precursor Cells; NG2: Neuron Antigen Glia 2
One of the most credited hypotheses on tumorigenesis is the origin of tumors from cancer stem cells (CSCs). These would be at the top of a cell hierarchy and responsible for growth, recurrence and resistance to therapies. There would be an equilibrium between CSCs and cancer non-stem cells through an interconversion from one to another [1]. The cell heterogeneity of malignant tumors would be, therefore, associated with a cell plasticity in which the bidirectional conversion would be modulated by specific microenvironmental signals arising in the tumor niches [2]. The interconversion hypothesis would reconcile the clonal evolution model with the hierarchical/cancer stem cell model [3]. It must be remembered that the tumor microenvironment is organized in niches where CSCs and mesenchymal stem/stromal cells are hosted and represent a target for therapies [4]. In gliomas, the stem cell hypothesis is a widely followed one. One of the first relevant observations was that the rat non-invasive normal neural stem cells (NSCs) become invasive if expanded with fibroblast growth factor 2 (FGF2) and bone morphogenetic protein (BMP4) and contain Ngfr, Sparc, Snail1, Pdpn and Tnc genes that are co-expressed in glioblastoma (GB) [5].
GB stem cells (GSCs), originating from the transformation of NSCs [6], share proliferation capacity and self-renewal with them and genetic/epigenetic alterations with GB [7]. Generally, with the term GSCs a heterogeneous pool of stem cells and progenitors is indicated, that could originate from stem cells by transformation, or from cancer cells by dedifferentiation [8-10]. GSCs would be a special cell population responsible for tumor growth, recurrence, resistance to radio- and chemotherapy and for the failure of the local tumor control. Recently, also oligodendrocyte precursor cells (OPCs) or so-called neuron antigen glia 2 (NG2)+ cells have been recognized as possible source of gliomas [11-13].
GSCs can increase in the tumor due to the increased symmetric self-renewal division rate or a reprogramming of non-CSCs to CSCs that confers them plasticity [14]. This is what happens after radiotherapy by activation of the DNA damage response [15] with expansion of the (more resistant) quiescent fraction of CSCs as they return to a proliferative status [16]. The CSC self-renewal may turn from asymmetric to symmetric division [15-18] and to a faster cell cycling [15]. The interconversion could be due to chemotherapy with Temozolomide that can shift non-GSCs towards GSCs that become positive for CD133, sex determining region Y-box 2 (SOX2), octamer-binding transcription factor 4 (Oct4) and Nestin [19]. Also epigenetic mechanisms may intervene in the equilibrium by bidirectional regeneration mechanisms of CSCs [1] further confirming the plasticity to the system [20]. The GSC status can be reached by dedifferentiation, mainly through hypoxia [21] or sub-toxic [22] irradiation [23]. We hypothesized that cell dedifferentiation could take place in the most malignant tumor areas under the influence of tumor microenvironment [24] with its crucial perivascular and perinecrotic niches [25].
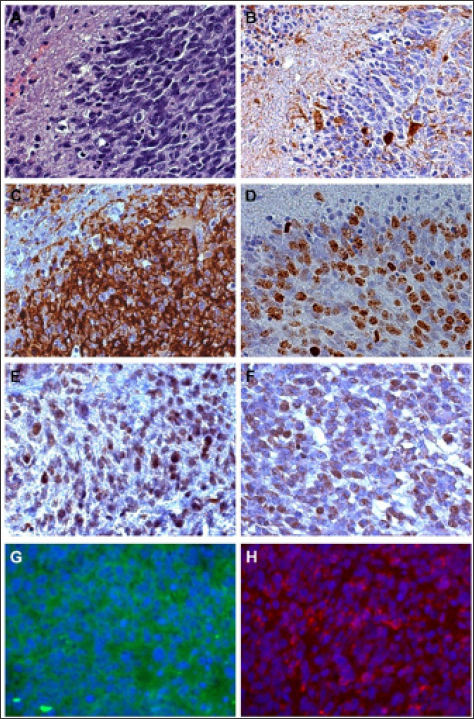
An immunohistochemical study demonstrated that, in GB, GSCs are characterized by NANOG, spalt like transcription factor 4 (SALL4), SOX2, pSTAT3 expression and low abundance of Oct4 at the protein and mRNA levels that would indicate a stem cell hierarchy (Figure 1) [26].
Figure 1: Immunohistochemistry. A - Glioblastoma hyperproliferative area bordering a circumscribed necrosis; H&E, x400; B - Id, negativity for GFAP that is limited to reactive astrocytes; DAB, x400; C - Intense positivity of Nestin in hyperproliferative area; DAB, x400; D - High Ki-67/MIB-1 labeling index in the same area; DAB, x400; E - Id, Intense SOX2 staining in several tumor cells; DAB, x400; F - Id, SELIL positive tumor cells; DAB, x400; G - Id, IF for CD133 and H - for Musashi-1; x400; Green and Red, respectively.
The whole matter had been long discussed with the conclusion for an interconversion [27]. In our experience, we demonstrated that:
i) in hyperproliferative areas of GB, usually at the border of the central necrosis, the Ki-67/MIB-1 labeling index and vascular density are the highest and circumscribed necroses occur. Most cells are Nestin+, SOX2+, SEL1L+, CD133+ and, therefore, oriented towards the high steps of the hierarchic scale of stemness [28,29];
ii) circumscribed necroses originate, as an alternative to a vessel wall pathology [30,31] with the pseudopalisades due to the cell escaping hypoxia [32], in avascular areas due to the imbalance between the high proliferation rate of tumor cells and the low one of endothelial cells [33]. The location of CSCs at their borders [34] is due to their sparing from the developing necrosis [29,35,36];
iii) not all tumor/vessel structures inside the tumor are a niche; the term should be reserved to those structures in which a direct contact between endothelial cells and GSCs is realized [35];
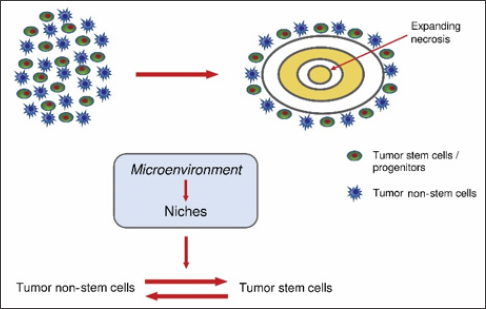
iv) genetic and epigenetic signaling influences the microenvironment that in turn conditions the GSCs/non-GSCs interconversion (Figure 2) [35,37].
Figure 2: Development of circumscribed necrosis (A). Equilibrium between tumor non-stem cells and tumor stem cells (B).
A review of the entire hierarchy of cells from GSCs to differentiated cells is compared with an ecological system where the microenvironment is remodeled by the cells and by its niches [38].
Everything considered, including hypoxia, microenvironment, epigenetic signaling, etc., it is very likely that in GB, after its origin from "tumor originating cells", an equilibrium is reached from time to time between stemness and differentiation with the consequent interconversion of cells between tumor stem cells/ less differentiated progenitors and tumor non-stem cells and more differentiated progenitors. Obviously, stem cell should be referred to a functional cell status and not to a cell type [39,40]. The awareness of the existence of this possibility could be of help in establishing therapies for GB.
This study was supported by the Grant n. 2016.AAI2705.U3302 from Fondazione Compagnia di San Paolo (Turin, Italy) and by Fondazione Cassa di Risparmio di Vercelli (Vercelli, Italy).


