Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Maria Milanova*1, Joana Zaharieva1, Aravin Pasula2, Nguyen Bach2, Ingolf Bernhardt2 and Dimitr Todorovsky1
Received: August 21, 2018; Published: August 28, 2018
*Corresponding author: Maria Milanova, Department of Inorganic Chemistry, Faculty of Chemistry and Pharmacy, University of Sofia "St. Kliment Ohridski", 1, J. Bourchier Blvd., Sofia 1164, Bulgaria
DOI: 10.26717/BJSTR.2018.08.001656
SiO2 films are deposited on microscopic glasses by dip- or spin coating using sol-gel produced starting solution. The morphology of the films is characterized by means of atomic force microscopy (AFM). The IR spectroscopy proved the presence of Si-OH groups on the surface of the films. The films as substrates for red blood cells in the present of fluoro-4 are studied by fluorescent microscopy. The shape change of the red blood cells can be connected with the IEP of the SiO2 and the influence of the hydrophilic surface of the thin glass film.
keywords: SiO2-Films; IR Spectra; Red Blood Cells
Under non-pathological conditions the human red blood cells (RBCs) can occur in different resting shapes - stomatocytes with "moon like" shape, discocytes with biconcave-disk shape, and echynocytes with protrusions or spicules. The normal discocyte is able to transform to either stomatocytes or echinocytes by changing the environmental conditions. Among different agents causing the modification of the discocytes to echynocytes both the proximity to a glass surface and high pH can be pointed out [1-3]. At physiological pH 7.4 the human RBC takes a biconcave discoid shape transforming into stomatocytes or echynocytes on decreasing or increasing of the pH value, respectively [1,4,5]. With the increase of the medium's ionic strength a red blood cell undergoes shape transformations in the sequence stomatocyte → discocyte → echinocyte [5,6]. According to [7-9] the shape change can be explained by the so called "bilayer couple mechanism". Measurements have shown that the variation of the incubation pH can alter the surface charge density of the RBC and thus can lead to a change of its membrane elastic properties, and subsequently its shape and size [5].
At the same time the glass surface is among the different materials used as support in a microscopic observation of processes such as the influx of Ca2+ in RBC [3]. The discocyte shape could only be preserved by moderately hydrophobic glass [2]. When RBCs get in contact with a glass or artificial surface a very fast shape change has been observed applying digital holographic microscopy [3]. The sol-gel process is very well adapted for thin glass films fabrication either by spin or dip-coating techniques.Produced by this way SiO2 - based films are often used to provide a microporous immobilization or supporting matrix with good adhesion, mechanical strength and excellent optical transparency. The preparation process involves hydrolysis and condensation of the alkoxide in the starting solution which produces a suspension of colloidal particles i.e. the sol. The following polycondensation leads to a rigid siloxane network, i.e. the gel, which after suitable thermal treatment forms a dry gel. In the work presented the performance of SiO2-based matrix (prepared by the above described method) as support for RBC observation is studied in order to find different substrates than a pure glass. The surface of the films is investigated in order to detect a possible interaction with RBCs and to observe the changes of the RBC shape.
Materials: Tetraethoxysilane, TEOS, >98 %, produced by Merck, and ethanol (p.a., 96 %) were used as initial materials. Glass microscope slides, cleaned sequentially using deionized water, methanol and acetone followed by a final deionized water rinse were used as substrates.
Starting Sol Production: Water-ethanol solution of the precursor (mole ratio ethanol: water: precursor = 4:1:0.25; pH = 1, adjusted by HCl) was prepared. The sol was stirred magnetically for 1 h and aged at 70 oC for 18 h to promote the hydrolysis and condensation prior to coating. Some of the sols were sonicated for 45 min after magnetic stirring by an ultrasound generator (Technopan UD-20). More details about the gel preparation method are described in [10].
Films Deposition: Films were deposited by dip-coating using a device, described in [11] at 0.4, 0.6 and 1 mm/s withdrawal rate at one immersion or by spin coating (spin coater KW-4A) at 20006000 rpm (67 - 604g) spinning rate and 20 to 60 s time of spinning. The films were stored in a dark box at ambient conditions until their use. In the experiments discussed in the present work films stored for 90 days were used. The thickness of the so produced films (1x1 cm), measured by a Talystep profilomer, depends on the deposition conditions and is typically around 300 nm after one immersion for dip-coated and 7 (0.5 mL gel, 3000 rpm (150g), 30 s) - 19 mm (1.5 mL gel, 2000 rpm (67g), 20 s) for spin-coated films. Their morphology depends on the deposition mode as well as, mainly, on the precursor nature [10].
Films Afm Characterization: Films surface was examined by atomic force microscopy (AFM device, Digital Instruments Nanoscope) with a resolution of 512x512 pixels, scan size between 0.5 and 10 μm, scan rate 0.4455 - 0.7535 Hz, resolution of the AFM tip less than 10 nm. Graphic (height mode) and viscoelastic (phase mode) data were recorded simultaneously in a tapping mode.
The human erythrocytes were obtained by washing the blood by centrifuging three times at 2000g for 5 min in physiological (high) ionic strength (HIS) solution containing 145 mM NaCl, 7.5 mM KCl, 10 mM glucose, 10 mM HEPES titrated with NaOH to pH=7.4. Intracellular Ca2+ levels of single cells were monitored by fluorescence microscopy using Ca2+ sensitive dye fluo-4 AM used as 1-2 μmol/L solution in dimethyl sulfoxid. Fluo-4 has a high affinity for binding Ca2+ and a large fluorescence intensity increase in response to Ca2+ binding. Washed RBCs (1% haematocrit) were incubated with the fluo-4 AM for 45 min at 37 °C in dark. Cells are then washed in physiological solution to remove any dye. After resuspension in the same buffer they are embedded on thin SiO2 films and observed with fluorescent microscopy
The fluorescent measurements were made by an inverted fluorescent microscope Nikon T - 2000 E. Magnification used was 600x. The dye molecules were excited with a xenon lamp at a wavelength of 488 nm and the emission was recorded at 520 nm.
The IR spectra of the surfaces of the SiO2 films were collected with a Nicolet 6700 FTIR spectrophotometer (Thermo Electron Corporation) accumulating 64 scans at a resolution of 4cm-1 and displayed in absorbance units in the interval 1200 - 700 cm-1 and 4000 - 400cm-1 with the purpose to follow the existence of OH- groups and Si-OH groups.
The contact angle of a water drop on a solid surface was measured with DSA10, Kruss GmbH using so called Tangent method 1 at working temperature 25±1 °C. The water phase was from Milipore system, with resistivity of ~ 10-15A and a water drop with a size of 200L.
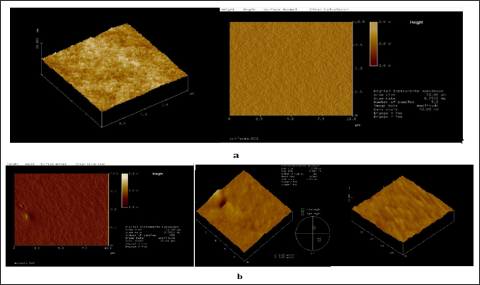
Morphology: The morphology of the substrates used can be evaluated from the AFM observation. Some examples of AFM- pictures are shown in Figures 1 & 2. The films are uniform with relatively small morphological grains. The decrease of the thickness as a result of the increase of the rate of withdrawal or spinning (Figures 1b & 2b) leads to slightly expressed wave-like surface. However, for films made by dip coating the distance between the highest and deepest points is smaller at withdrawal rate 0.4 mm/s (Figure 2b) than at withdrawal rate 0.2 mm/s (Figure 2a) and less than 5 nm.
Figure 1: AFM measurements of SiO2 based films produced by spin coating, 30 s spinning at 2000 min-1 (67 g) (a) and 3000 min-1 (150 g) (b).
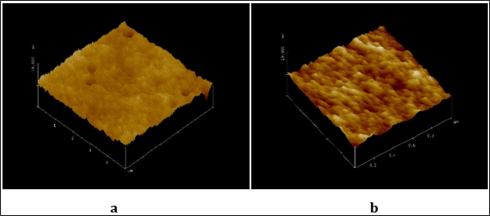
Figure 2: AFM measurements of SiO2 based films produced by dip coating at withdrawal rate (mm/s) 0.2 (a) and 0.4 (b).
IR Spectra: A broad absorption band at 2500-3400cm-1 is well expressed in the IR spectra of the films investigated, with a maximum at about 3350cm-1 (Figure 3a). At the same time, it is not observed in the IR spectrum of the pure glass (Figure 3a). The band could be caused by the presence both of water molecules and OH groups [12]. Taking into account that no absorption around 1625cm-1 is observed, i.e. no bending of H2O [12], it can be concluded that mainly OH groups contribute to the absorption bands in the interval 2500-3400cm-1-The absorption of the OH groups is probably connected with the broad band at 980cm-1 as well, due to Si-OH stretching mode [13], like the one observed in the IR spectrum of the SiO2 film with 1 mm/s withdrawal speed (Figure 3b). The absorption band is shifted to higher wavenumber (1020 cm-1) for the films made at 0.4 and 0.2 mm/s withdrawal rate (Figures 3b & 4). So, the presence of the OH groups can be considered proved by the IR spectra although O-Si-O asymmetric stretching also can be ascribed to band around 980cm-1 [14].
Figure 3: IR spectra of dip-coated films in 4000 – 400 (a) and 1200 - 700 cm-1 (b), at a different withdrawal rate: glass substrate (1), 1 mm/s (2), 0.4 mm/s (3); 0.2 mm/s (4).
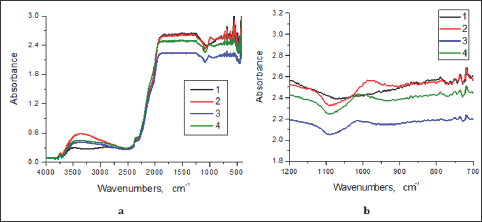
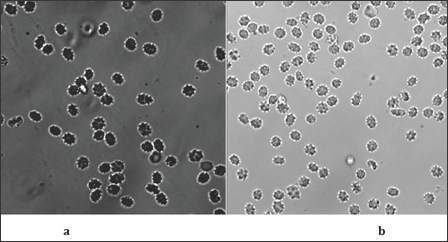
Figure 4: Fluorescent images of echynocytes on films produced by dip-coating at withdrawal rate of 0.4 mm/s (a) and by spin-coating (30 s, 3000 min-1, 150 g) (b)
The broad shoulder observed at about 1120 cm-1 (Figures 2-4) could be due to Si-O-Si network formation, although the vas (Si-O-Si) band appearance could be at 1220 cm-1 [15]. The shift to the lower frequency of the band for Si-O-Si in our samples could be explained [16] with the superposition of the Si-O(C) absorption band at 1150 cm-1 due to the presence of partially non-hydrolized TEOS- fragments. The absorption band with a low intensity noticable at 795cm-1 (Figure 3b) could be ascribed to O-Si-O [14]. In the same time some trimers and tetramers, (HO)3SiO (HO)2SiOSi (OH)3 and [(HO)2SiO]4, can be formed [17]. The presence of three types of Si-atoms and formation of similar groups HOSiO3, (HO)2SiO2 and HOSiO2OC2H5 bonded in cyclic tetramers are suggested and proved by elemenal analysis in [16] where the synthetic procedure used is like the one in the work presented. The cyclic tetramers of the type C2H5OSi4Ox (OH)y.H2O can be the main part of a gel made of TEOS- containing sol. Such formations give an option for and facilitating of the arrangement of the RBC on the matrix.
Contact Angle: The contact angle measurements show equal values for the SiO2-films obtained at different withdrawal speed (Table 1). In all cases observed the water droplets exhibit contact angles corresponding to a hydrophilic surface obviously due to the presence of OH and Si-OH groups proved by the IR spectra above. It is obvious that our samples are more hydrophilic than the glass substrate used.
The RBC - Image on the Surface: All three types of red blood cells echynocytes (Figures 4a & 4b), discocytes (Figure 5a) and stomatocytes (Figure 5b) are observed by fluorescent microscopy on the films presented above as supporting substrates. The hydrophillicity is main difference between the surface of the prepared samples and the glass substrate. Apparently echynocytes can be observed equally satisfactory on films, produced both by dip- (Figure 4a) and spin- (Figure 4b) coating. The withdrawal rates of 0.4 and 0.6mm/s of the films produced by dip-coating do not create a difference significant enough in order to make a difference on the discocytes and stomatocytes observed (Figure 5). It is difficult to find a connection between the morphology and the surface of the films and the RBCs type observed on that surface. The fluorescent image of discocytes and echynocytes on a film produced by dip- coating at withdrawal rate of 0.6 mm/s could be considered as a proof for the transformation of RBCs (Figure 6a & 6b). It can be said that the procedure of synthesis of SiO2 films using TEOS as an initial substance is the reason for the existence on the surface of SiO2-films of OH groups. The latter are proved by the IR spectra. At the same time the pH of the buffered RBCs (7.4) is far above the isoelectric point for the SiO2 (1.7-3.5). When the RBCs solution gets in contact with the film predominant surface species like Si - O- can be formed causing a net negative charge of the surface. That can be responsible for the RBC positioning on the surface since surface of RBC has negative charge [18].
Figure 5: Fluorescent images of discocytes (a) and stomatocytes (b) on films produced by dip-coating at withdrawal rate of 0.6 mm/s (a) and of 0.4 mm/s (b). f 0.4 mm/s (a) and by spin-coating (30 s, 3000 min-1, 150 g) (b).
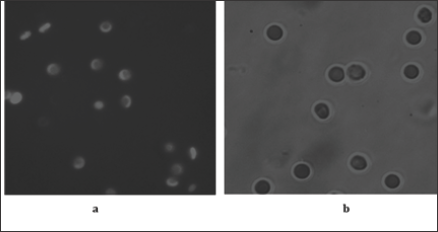
Figure 6: Fluorescent image of discocytes and echynocytes (a) on a film produced by dip-coating at withdrawal rate of 0.6 mm/s (b).

The SiO2-based films made by us with TEOS via sol-gel method show resonable OH (respectively Si-OH) surface concentration. In comparison with the glass substrate the films are more hydrophillic. All the types of red blood cells were observed on the hydrophillic surface of the films, including discocytes. The easy arrangement of the RBC on the films could be due to both the Si-OH groups and the formations of trimers and tetramers suggested on the base of IR spectra and literature data. A modification of the discocyte to echynocyte in the proximity to a glass surface was observed on a dip-coated film with 0.6 mm/s withdrawal rate. It can be expected that films synthesised by organically modified silanes (MtEOS, methyltriethoxysilane, OtEOS, octyltriethoxysilane,) or mixture of TEOS + OtEOS, showing a hydrophobicity of the surface, could contribute in a different way to the RBC shape. If the properties of the glass surface, namely its OH groups, are important for the modification of RBC as it is stated in [8] the acidity of the surface has to be followed.
The work is performed with the financial support of the Bulgarian Fund for Scientific Investigation (contract VUH 05/2005) and the bilateral agreement between University of Sofia and Saarland University. Contact angles were measured in the Department of Chemical and Pharmaceutical Engineering, University of Sofia. The IR spectra are recorded in the Institute of Catalysis, BAS.


