Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
J Cancino Bernardi
Received: August 17, 2018; Published: August 24, 2018
*Corresponding author: J Cancino Bernardi, Nanomedicine and Nanotoxicology Group, Physics Institute of São, University of São Paulo, Brazil
DOI: 10.26717/BJSTR.2018.08.001634
The application of nanotechnology in the medical field has been growing year by year, reflecting the advances of nanomaterials as theranostic agents for the detection and treatment of diseases, especially in oncology. However, there are several barriers that inhibit the efficiency in the use of these materials for medical applications, for example, opsonization and phagocytosis processes, low biodistribution, and especially, lack of specificity and selectivity. Natural cell membrane nanoparticle coating is one of the newest and most innovative strategies for solving such problems by offering highly selective surfaces that are difficult to achieve using traditional synthetic products. Combined with advances in nanoparticle camouflage are drug delivery and therapies, as photothermal, which makes use of the surface plasmon resonance effect of nanoparticles to generate a rapid localized heating, ideal for favoring the death of cancer cells. In this context, the emphasis of this mini review is presenting some successful research in theranostic nanomaterials camouflaged with cell membranes for therapies and delivery.
Abbreviations: NPs: Nanoparticles; PLGA: Poly Lactic Co Glycolic Acid; PLA: Poly Lactic Acid; Dox: 30 Doxorubicin; HRBC: Human Red Blood Cells; MSC: Mesenchymal Stem Cells; PT: Photothermic Therapy; LSPR: Localized Surface Plasma Resonance; AuNCs: Gold Nanocages
In the last years, numerous studies have focused on improving nanomaterials to be applied as theranostics agents for cancer therapy; some design strategies to obtain this was illustrated in Figure 1. But the therapeutic efficacy of nanomaterials is still a challenge in nanomedicine due to poor delivery route and biodistribution. It is a consensus in the area, no matter what type of surface functionalization, active or passive, more than 99% of nanoparticles administered invivo are phagocytized by the biosystem. However, these problems have been apparently solved by the use of natural cell membrane coating the surface of nanomaterials. Some of this enthusiasm came from the results obtained in terms of biodistribution, selectivity, and specificity. The nanoparticle with natural cell membrane is one of the most innovative strategies and has surprising results [1]. The NPs cell membrane coating has been extended to cancer cells therapies and has also shown great potential as drug delivery systems. With this approach is possible to overcome the immune system barriers and enhance the accumulation of nanoparticles in the tumor; moreover, it had demonstrated increasing specificity for cancer cell targeting and lower tendency to be eliminated by the immunological system [2]. In this brief review, it is presented some of the hot studies in the area.
Figure 1: Different cell membrane-based strategies for nanoparticle functionalization. (A) Cell 51 membrane coating as a promising target. (B) Innumerous biomolecules to mimicking membrane 52 recognition. With permission from Ref Fang et al. [2].
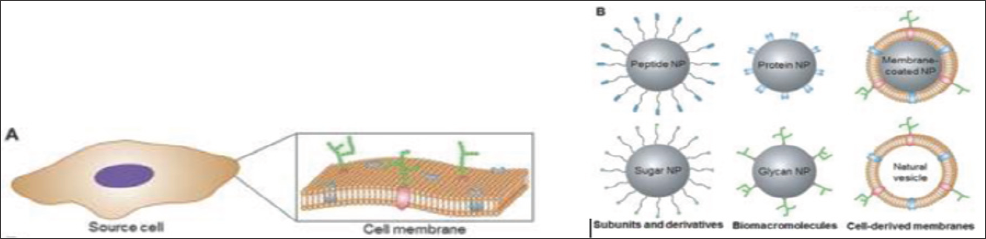
Polymeric-based particles look to be a standard in this kind of modification, the major studies that coated the NP surface used PLA or PLGA. One of the first studies using this strategy reports a polymeric nanoparticle with natural erythrocyte membranes for delivery. In vivo results showed that nanoparticles promoted superior circulation half-life and a significant retention in the blood even 72h after their injection. These results were associated with the membrane proteins and biomolecules behavior at the nanoparticle surface [3]. After that, some studies had been devoted to improving and understanding the advantages of cell membrane coating to delivery some compounds. In a recent study, the delivery of doxorubicin was potentialized by human umbilical cord-derived mesenchymal stem cell (MSCs) membrane coating on dox PLGA based NPs. The membrane functionalization showed exceptional tumor growth inhibition, probably due to the significantly enhanced uptake, tumor-targeting, and accumulation of the chemotherapy- NPs into cancer cells [4].
Membranes of human red blood cells (HRBCs) were coated onto polymeric NPs to act as biomimetic toxin sponges to absorb and neutralize hemolytic toxins. Those particles were considered an excellent antivirulence platform against hemolytic toxins like a-hemolysin of methicillin resistant Staphylococcus aureus, listeriolysin O of Listeria monocytogenes, and streptolysin O of Group A Streptococcus. Interesting those membranes-based NPs do not show in vitro cytotoxicity or in vivo lethality which indicates effective toxin neutralization and specificity [5]. A dual membrane NP coating was designed to offers a stem cell therapy to patients with acute liver failure. For this, mesenchymal stem cells were used as NP core whereas membranes of red blood cells as NP shell, both membranes were applied to carry regenerative factors and to increase blood stability, respectively. In vivo study indicated exciting results as an increase in liver cell proliferation and lower internalization by macrophage cells. Along with the circulating levels of pro-inflammatory cytokines were reduced, the liver of the mice was regenerated, improving the survival rates of the animal in acute liver failure [6].
Allied to nanoparticles cell membrane coating there are some therapies that can improve even more the efficacy of this strategy. Photothermic therapy (PT) is one of the promising techniques that can be associated with NP-based cell membranes. PT is able to convert the light absorbed by the metallic NP into heat, generating a rapidly localized heating ideal to support the death of cancer cells. The novelty of this type of treatment is a 84 possibility of nanomaterials promotes heating and delivery together in a synergy way. Gold based NPs are known to induce this heating due to their localized surface plasma resonance (LSPR) ability. Gold nanocages (AuNCs) coated with RBC membrane demonstrated significant and selective in vitro photothermal effect upon NIR laser irradiation on cancerous cells.
The synergy offered by photothermal effects from AuNCs and long blood circulation lifetime from RBCs, enhanced tumor uptake and achieve 100% survival mice over 45 days after treatment [7]. Selenium NPs combined with a chemical bevacizumab, both coated with RBC cell membrane showed as a potential cell membrane- based NP for simultaneous radiotherapy and antiangiogenic therapy of cancer. The RBC membrane coating prolongs the blood circulation time and reduces their elimination by the immune system. The results from the irradiation of cell membrane-based NPs with X-rays demonstrated potent anticancer and antiangiogenic responses evidenced by tumor growth inhibition in nude mice, without causing any histological damage to the non-target major organs [8].
Cell membrane-based NPs is the next generation of theranostics nanomaterials. It seems to be an excellent alternative to overcome some biological barriers, especially in terms of the immune response, blood circulation time and stability. Moreover, the biomolecules extracted together with the lipids from the membrane can promote an unusual selectivity to the nanomaterials, increasing their efficacy in some therapies.


