Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Oded Meiron1*, Julia Namestnic2, Jonathan David1, Annaelle Dynovisz1 and Rena Gale2
Received: July 01, 2018; Published: August 20, 2018
*Corresponding author: Oded Meiron, Herzog Hospital, Givat Shaul Street, Jerusalem 91035, Israel
DOI: 10.26717/BJSTR.2018.08.001614
The study aimed to identify significant changes in specific paroxysmal EEG features at early infancy stages as indicators of late-infancy epileptic foci formation in an infant suffering from early-onset epileptic encephalopathy. Early infancy EEG was recorded for 90 minutes at ages four, and seven months. At late infancy, video-EEG was recorded for 8 hours at age 23 months. Early and late-infancy EEG data was visually analyzed for seizure-related epileptic discharges, including the assessment of slow-wave spikes frequency and their topography at early infancy, and identification of seizure- related spectral power density changes, and their most dominant semiology, at late-infancy. Statistically significant age-dependent differences in interictal slow-wave-spike frequency at parietal cortex locations during early-infancy were consistent with parietal cortex epileptic foci dominance at late infancy. The results support developmental age-dependent EEG assessments in detecting significant paroxysmal location-specific changes of slow-wave spike frequency in early-infancy as indicators of epileptic foci-dominance and progression in early-onset epileptic encephalopathy. Replication of our findings in other neonatal electroclinical syndrome cases that suffer from seizure intractability is likely to propagate early focal treatment interventions to inhibit location-specific paroxysmal epileptiform activity in neonatal catastrophic epilepsies.
Keywords: Suppression Burst Pattern; Interictal Spikes; Age Dependent Epileptic Encephalopathy; Epileptic Foci; Seizure Intractability; Spectral Power Density
Abbreviations: EEG: Electro Encephalography; SB: Suppression Burst Pattern; AES: Age Dependent Epileptic Encephalopathy Syndromes; EPF: Epileptic Foci; OS: Ohtahara Syndrome
Infant cases of early-onset epileptic encephalopathy syndromes suffer from frequent continuous intractable seizures and may be phenotypically characterized by an electroencephalography (EEG) suppression-burst pattern (SB) [1,2]. A large proportion of age-dependent epileptic encephalopathy syndrome cases may demonstrate a persistent SB and are often classified as Ohtahara syndrome [1]. Newborns diagnosed with Ohtahara syndrome (OS) or other Age dependent epileptic encephalopathy syndromes (AES) (e.g., West syndrome) display paroxysmal epileptiform activity throughout their development associated with structural abnormalities and age-dependent brain dysgenesis. Characteristic pathological EEG features observed in OS are SB's, which include high-voltage bursts with alternating flat "suppression" activity in both awake and sleep periods [1,3]. Ictal and interictal EEG during the developmental course of early-onset electroclinical syndromes can help monitor disease progression, and the development of region-specific epileptic foci dominance, thus; tracing the progression of pathological EEG semiology may contribute to the diagnosis of different electroclinical syndromes, but more importantly may predict disease progression and corresponding epileptic foci (EPF) formation [1,4].
The current study aimed to assess specific early-infancy abnormal epileptiform EEG parameters to identify possible region- specific paroxysmal EEG features, [5,6] such as the frequency of paroxysmal high-voltage interictal-slow-wave spike discharges associated seizure frequency and progressive organic abnormalities [1,3]. Accordingly, in response Ohtahara and Yamatogi empirically- driven theoretical approach suggesting that age-specific EEG longitudinal assessments in early-onset epileptic encephalopathy indicate the manifestation of new electroclinical features, 1 we hypothesized early infancy location-specific changes in SWS frequency could predict late-infancy topography of epileptic foci progression and dominance.
The case we studied was a male infant, born after an uneventful, full term pregnancy with birth weight of 3,160 g through a normal vaginal delivery, to heathy young parents with an older healthy child. The infant was discharged and re-hospitalized at the age of five days due to feeding problems. Nine days after birth magnetic resonance imaging revealed no brain abnormalities. Upon re-admission at age six weeks repeated clinical seizures with bradycardic spells and desaturation appeared. Initially, he was treated with Phenytoin and Phenobarbital with no apparent effect. Preliminary EEG recordings between ages two to four months revealed a consistent SB pattern along with high seizure-frequency and seizure intractability, and OS was suspected. Metabolic disorders were excluded. Consequently, the antiepileptic drugs were changed to Clonazepam 0.8 mg/day, vigabatrin 500mg/day and topiramate 60 mg/day, however, the seizures continued and were complicated by repeated aspirations and baby required continuous respiratory treatment. Eventually, topiramate was increased to 100 mg/day. In general, over time the seizures subsided in intensity and frequency. The infant suffered less from aspirations; however, he could not be weaned from the respirator. There were no apparent signs of motor developmental milestones. He gained weight throughout his infancy period and weighed 17 kg at age 23 months.
Early-infancy EEG acquisition periods utilized a 14 channel EEG system (Emotiv EPOC, San Francisco, U.S.A) and the late-in- fancy EEG recording utilized a 32-channel video-EEG system (ANT Neuro, Netherlands). Early-infancy EEG sessions were recorded for 90 minutes during wakefulness. We noted early infancy epileptiform EEG activity including ictal events (IcE) at ages four and seven months. Late infancy video-EEG was recorded at age 23 months, for eight hours, including four hours of wakefulness and four hours of sleep. Visual inspection of early infancy EEG examined the number of paroxysmal (amplitude-increase over ± 400 m V) interictal SWS during burst-periods associated with seizures, and, assessed changes in the duration of burst-periods associated with seizure onset. Visual inspection of late-infancy video-EEG was conducted to detect and mark seizure-onset events. In order to attain late-in- fancy region-specific changes in seizure-related spectral EEG activity, we computed seizure- related changes in delta, theta, alpha and beta band spectral-density power immediately following the onset of the seizure versus pre-seizure time-window (by computing the averaged SDP differences between a pre-seizure-onset 3000ms time window and a post-seizure onset 3000ms time-window, with 250ms offset before and after seizure-onset) [7]. Seizure-related analysis of maximal changes in spectral density power (SDP) provided an averaged estimation of band-specific energy peaks under certain electrodes within a seizure-related time-window. [8] Seizure-related differences in SDP from pre to post-seizure-onset window were obtained by running an averaged fast Fourier Transformation (FFT) in 500ms steps across 6000ms epochs of seizure-related events.
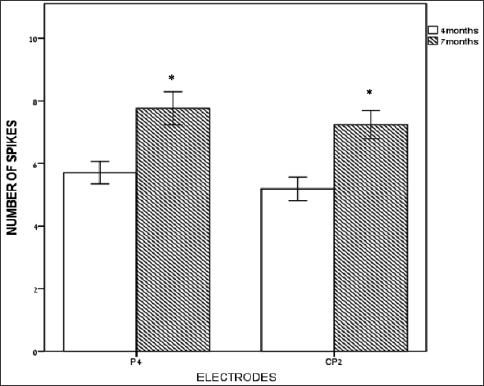
The number of high-voltage paroxysmal interictal slow-wave spikes (SWS) during the ictal burst periods was significantly higher at age 7 months versus age 4 months (Figure 1), only at right parietal electrodes P4 {t(14) = -3.189, p = 0.007} and CP2 {t(14) = -4.079. p = 0.001} versus all other electrode locations. Spike amplitudes and spike durations were not significantly different at 7 months versus 4 months.
Figure 1: Number of slow wave spikes (SWS) at early-infancy under right parietal electrodes. Number of paroxysmal high- voltage slow-wave spikes (Y axis) at right central-parietal-CP2 and right parietal-P4 electrodes (X axis) at 4 months and at 7 months. Bars represent the mean scores, and error-bars are +/- 1 SE. * represents a significant difference (P < 0.05).b
Visual inspection of video-EEG detected 227 seizure-related events. Seizure related (time-locked to seizure-onset) increase in delta-band SDP (i.e., delta synchronization) was observed at left parietal-occipital area spanning from P3 to P7 electrode locations (Figure 2). Seizure-related increase in beta-band SDP was also observed at the left parietal P7 electrode location (Figure 2). Seizure related decrease in alpha-band SPD (i.e., alpha desynchronization) and in theta-band SPD were observed over an area spanning CP6 to P8 electrode locations (Figure 2), corresponding with further lateralization of observed early infancy changes in SWS frequency at P4 and CP2. Late-infancy seizure-related increase in delta-band SPD showed the largest proportional maximal change in SDP versus the other bands (Figure 2), which was often paired with tonic spasms of contralateral right arms or legs (approximately 70% of seizures) as captured by video-EEG [8]. As indicated by Figure 2, an increase of left parietal delta and beta SDP was paired with a decrease in right parietal theta and alpha SPD across all 227 marked seizures. The contralateral changes in SPD per frequency-band are not shown in Figure 2, as we were interested in showing only the largest paroxysmal changes during seizures which usually occurred over the left or right hemisphere. The main seizure patterns at late infancy were tonic spasms. Helsinki committee approval and informed consent were obtained prior to study initiation. Follow- up visits indicated that the infant has survived past the age of 24 months.
Figure 2: Seizure related changes in spectral density power (SDP) head-maps at late-infancy. SDP scale (in m V2/Hz) can be viewed on the left (see color bars) where red denotes an increase in SDP and blue denotes a decrease SDP. Top: left side showing seizure-related delta (0.5-3.5 Hz) SDP increase at left parietal P3 and P7 electrodes, and at the top right theta-band (3.5-7.5 Hz) seizure-related SPD decrease can be observed under right parietal CP6 and P8 electrodes. Bottom: head-maps showing seizure-related SDP decrease in alpha-band (7.5-12.5Hz) at right parietal electrodes (bottom left), and seizure-related beta-band (12.5-30Hz) SPD increase at left parietal electrodes (bottom right). The maximal (red) and minimal (blue) SPD values can be viewed at the left side of each SPD head map. At the top of each figure the number of epochs (1362) and the time interval (500ms) used to average the SPD changes across 6 second epochs, and for computing the difference in SDP (the word "difference" between the top values indicates this type of event-related analysis) between post and pre-seizure SDP three second time windows.
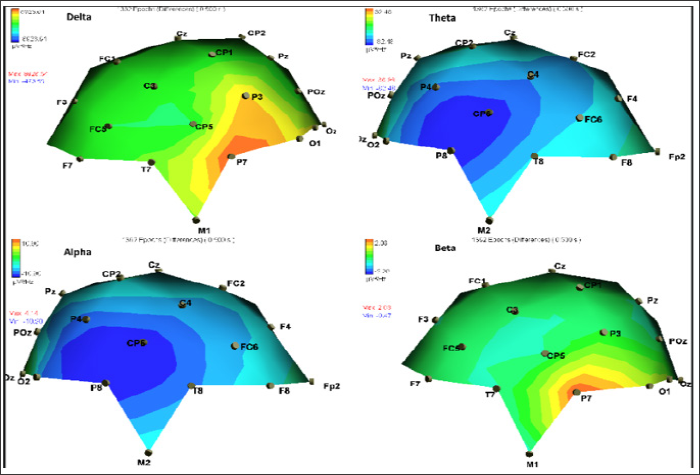
Significant age-specific changes in the number of interictal SWS observed only for right parietal electrode locations at early infancy predated location-specific right parietal lateralization of seizure- related alpha and theta paroxysmal desynchronization. Seizure- related delta and beta synchronization at the left hemisphere paired with seizure related theta and alpha right hemisphere desynchronization (Figure 2) could reflect pathology- related asynchrony progression at late infancy [9]. Therefore, as predicted, increased interictal SWS asymmetry within the same cortical area was observed at early infancy and was retraced as paroxysmal changes in seizure-related theta-band and alpha-band SDP at right parietal electrode locations at late-infancy. However, maximal seizure-related delta and beta band SDP indicated a left-right hemisphere asymmetry of epileptiform activity at late-infancy, versus right-left SWS activity at early infancy, lateralized to the right parietal-occipital electrodes, supporting an early infancy parietal dominance of EPF. The ongoing left-hemisphere epileptiform activity lateralization (to right and left hemisphere over the parietal cortex) observed at late infancy, along with emerging left-hemisphere paroxysmal fast-wave beta-band activity could hypothetically underlie an ongoing age-dependent pathological progression of EPF lateralization, associated with further "release" of the brainstem from cortical inhibitory control [3].
Overall, age-dependent EEG data collection over a period of 20 months in a case of neonatal epileptic encephalopathy revealed that changes in paroxysmal focal interictal spikes over time may supply a reliable prediction concerning the progression and formation of EPF. Specifically, we may have identified developmental paroxysmal EEG features, [1,9] such as focal (e.g., parietal cortex) seizure-related changes in asymmetrical delta and beta SDP, as an indication of EPF dominance. However, this novel EEG-based topographical analysis method for EPF detection, linking early SWS topography with later progressive lateralization of epileptiform activity, would need to be validated in other electroclinical syndrome cases. Replication of our findings might support future efforts towards the development of early focal non-invasive electrotherapies to significantly alleviate the frequency of seizures in early-onset catastrophic epilepsies [10,11].


