Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yuko Tada and Phillip C Yang*
Received: July 01,2018; Published: August 17,2018
*Corresponding author: Phillip C Yang, Stanford University School of Medicine, 300 Pasteur Drive, Stanford CA94305, USA
DOI: 10.26717/BJSTR.2018.08.001608
Abbreviations: DEMRI: Delayed Gadolinium Enhancement MRI; STEMI: ST Elevation Myocardial Infarction; AMI: Acute Myocardial Infarction; LV: Left Ventricular; IC: Infarct Core; PIR: Peri Infarct Region; IR: Ischemia Reperfusion Injury; MVO: Microvascular Obstruction; IMH: Intramyocardial Hemorrhage; T2WI: T2 Weighted MRI; ECV: Extra Cellular Volume; MEMRI: Manganese Enhanced MRI
While the advances in revascularization therapy have drastically decreased the mortality following ST elevation myocardial infarction (STEMI), the number of patients who develops heart failure (HF) following the reperfusion therapy continues to rise. More than 30% of the patients who survive STEMI develop HF long term [1]. In addition, patients with a history of acute myocardial infarction (AMI) and left ventricular (LV) dysfunction have a markedly increased 6 months mortality of greater than 10% [2] Thus, there is an urgent unmet clinical need to clarify the mechanisms of SCD and HF post-MI and develop a robust biomarker to identify the high-risk patients. The importance of differentiating the peri- infarct region (PIR) from the adjacent non-viable infarct core (IC) has been noted recently as it could independently determine the occurrence of major adverse clinical events [3,4]. The PIR consisting of heterogeneous tissue components represents the nexus for active remodeling or repair characterized by extracellular matrix formation, angiogenesis, oxidative stress, mitochondrial energetics, apoptosis and inflammation [5,6]. We here review the non-invasive MRI approaches to characterize PIR.
Delayed gadolinium enhancement MRI (DEMRI) is the current gold standard to assess the non-viable myocardial infarct. However, up to 10% of the DEMRI positive myocardial infarct includes histologically viable PIR represented by reduced signal of the gadolinium based contrast agent [7-9]. The ischemia reperfusion injury (IR) results in the formation of heterogeneous tissues, containing vulnerable cardiomyocytes in the PIR9. However, the exact relationship between the IR injury and formation of PIR remains unknown. The pathological changes associated with IR injury such as myocardial edema, microvascular obstruction (MVO) and intramyocardial hemorrhage (IMH) delineated by DEMRI and
T2 weighted MRI (T2WI) correlate with the major adverse cardiac events (MACE) [10,11]. Their predictive capability of LV arrhythmia and SCD in the post MI period has not yet been determined. Recent advances in T1 mapping technique could help characterize the PIR more precisely. Pathological changes, including fibrosis, necrosis and edema increase pre-contrast (native) T1 value of the myocardium. It contains both myocytes and connective tissue information. Expansion of the extra cellular volume (ECV) fraction in the infarct injury region causes a greater change in the MRI relaxation (R1) value after the administration of the gadolinium- based extracellular contrast agent when compared to the remote myocardium [12]. The ECV of the myocardium is calculated by measuring Hct and T1 for the blood pool: ECV = (1-Hct) x (R1post- R1pre) myo/ (R1post-R1pre) blood, where R1 equals 1/T1 [13]. Diagnostic values of native T1 and ECV in the acute myocardial infarction in predicting LV dysfunction have been indicated in several clinical studies [14-16]. However, one of the intrinsic disadvantages is that the ECV does not detect the dysfunctional myocardium directly.
An alternative approach, manganese enhanced MRI (MEMRI), uses manganese (Mn2+) based biological contrast agent to represent the viability of cardiomyocytes directly through the ability of individual cells to take up manganese ions through L-type calcium channels [17]. MEMRI distinguishes the injured but viable myocardium in the infarct tissue to assess the extent of salvageable myocardium in the heterogeneous PIR. We previously showed that the PIR can be delineated by the dual MEMRI and DEMRI contrast where the MEMRI positive viable area is located inside the DEMRI positive myocardial infarct [18,19]. DEMRI positive total infarct can be divided into non-viable MEMRI negative infarct core (IC) and viable MEMRI positive PIR territories (Figure 1). In addition, T1 mapping of the native and MEMRI-enhanced myocardium could delineate and quantitate the viability of PIR. Furthermore, the rapid first pass uptake of manganese by the cardiomyocytes measures the R1 change (delta R1) before and after MEMRI to represent the specific viability of the cardiomyocytes [20,21]. Thus, myocardial injury in the heterogeneous infarct zones can be assessed based on the differential myocardial cellular viability using T1 mapping before and after MEMRI [22].
Figure 1: PIR characterization using DEMRI, MEMRI and T1 mapping.
Note: The LAD IR injury model was created in a swine and MRI was performed 4 weeks later. DEMRI positive total infarct was divided into non-viable MEMRI negative infarct core (IC) and viable MEMRI positive PIR (yellow).
T1 mapping was performed using smarT1map sequence (GE Healthcare) before and after MEMRI. Native T1 and post-MEMRI T1 of each infarct territory can be calculated.
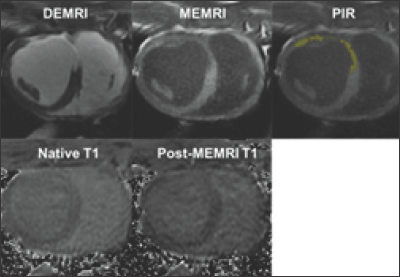
The viability signal may indicate LV remodeling and arrhythmia potential more strongly compared to the assessment of necrosis or fibrosis by native T1 or ECV mapping. Clinical trials in ischemic cardiomyopathy patients are on-going in our institution. Our preclinical study using swine IR injury model characterized the heterogeneous infarct tissues consisting of the infarct core (IC) and PIR based on native T1 value and myocardial viability. The analysis revealed IC, PIR, and remote zone all had distinct native T1, post MEMRI T1, and delta R1 values. Utilizing these novel contrast techniques, roles of IR injury in the formation of inflammatory, heterogeneous PIR, resulting in eventual MACE will be elucidated and a reliable non-invasive imaging modality to predict the occurrence of ventricular arrhythmia will be developed.
Characterization of myocardial viability in the PIR is feasible using T1 mapping post MEMRI. Unique strengths of each contrast agent must be leveraged to better understand the role of the PIR in HF and SCD.


