Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Teruko Honda*1 and Hiroyuki Inagawa2.3
Received: July 24, 2018; Published: August 02, 2018
*Corresponding author: Teruko Honda, Department of Medical Technology, School of Life and Environmental Science, Azabu University, Kanagawa, Japan
DOI: 10.26717/BJSTR.2018.07.001522
Introduction: Monocytes infiltrate tissues and differentiate into tissue-specific macrophages by interaction with other cells in tissues. Macrophages in the arterial wall uptake of oxidized LDL and form foam cells and induce inflammatory changes in tissues by secreting inflammatory cytokines. Chronic inflammation is believed to be involved in the development of cancer and lifestyle-related diseases. Whereas, in human monocytes, the mRNA expression of inflammatory factors increases by interactions with cancer cells however, this increase can be suppressed by pretreatment with low-dose LPS. In the present study, we investigated changes in the gene expression of some key cytokines, inflammatory factors [IL-1β and adiponectin] and a chemotactic factor [MCP-1], after interactions between human adipocytes and LPS-pretreated human monocytes.
Materials and Methods: The human monocyte cell line THP-1 was treated with LPS and subsequently co-cultured with human adipocytes using an insert co-culture system. The gene expressions of inflammatory factors and chemotactic factor were analyzed using quantitative real-time PCR and DNA microarray.
Results: The increased mRNA expression of IL-1β in human adipocytes after co-culture was suppressed by interaction with LPS-pretreated THP-1 cells. The decreased mRNA expression of adiponectin in human adipocytes after co-culture was increased by interaction with LPS-pretreated THP-1 cells. In addition, the increased mRNA expression of MCP-1 in THP-1 cells after interaction with human adipocytes was suppressed by LPS-pretreatment.
Conclusion: LPS-pretreated human monocytes may have anti-inflammatory effect in adipose tissues. LPS-treated human monocytes may be beneficial for the prevention of diseases caused by chronic inflammation.
Keywords: Monocyte, Adipocyte, Co-Culture, Lipopolysaccharide, Inflammatory Factor
Abbreviations: LDL: low density lipoprotein; LPS: lipopolysaccharide; IL: interleukin; MCP-1: Monocyte Chemotactic Protein-1; PCR: Polymerase Chain Reaction; VEGF: Vascular Endothelial Growth Factor; TNF: Tumor Necrosis Factor
Monocytes are a type of leukocyte that play an important role in the phagocytosis of pathogenic microorganisms and maintenance of homeostasis. Although monocytes are typically found in the blood, they may infiltrate tissues and differentiate into macrophages [1,2]. It is known that macrophages interact with other cells in tissues and take on tissue-specific functions and morphologies [3]. It has been shown that tumor-associated macrophages infiltrated tumor tissues promote metastasis and invasion of cancer [4-7]. Furthermore, it has been shown that macrophages infiltrated the arterial wall uptake of oxidized LDL and form foam cells [8]. Foamy macrophages in the arterial wall induce inflammatory changes in tissues by secreting inflammatory cytokines [9-11].
In recent years, it has become clear that chronic inflammation is involved in the development of cancer and lifestyle-related diseases such as diabetes, stroke, and arteriosclerosis [12,13]. Therefore, macrophages infiltrating tissues may be key cells in the immune response.
LPS is an extracellular membrane component of gram-negative bacteria. When a high concentration of LPS is intravenously administered, it causes severe systemic inflammation and acute septic shock [14]. On the other hands, it has been reported that environmental exposure to LPS in childhood might have an important role in the development of tolerance to ubiquitous allergens [15]. Previously, we reported that the mRNA expression of inflammatory factors in human monocytes increased by interactions with cancer cells and that this increase can be suppressed by pretreatment with low-dose LPS [16-18]. These findings suggested that LPS-treated human monocytes regulate inflammatory reactions. Whereas, it has been shown that inflammatory changes observed in adipose tissues with visceral obesity contribute to the development of metabolic syndrome [19-22]. Oral or transdermal administration of LPS has been shown to induce decreases in fasting blood glucose and LDL cholesterol levels [23-25]. LPS treatment on monocytes/ macrophages may change inflammatory reactions in adipose tissues. Here, we investigated changes in the gene expression of inflammatory factors, IL-1β and adiponectin, and chemotactic factor, MCP-1, after interactions between human adipocytes and LPS-pretreated human monocytes.
Cells THP-1 cells obtained from DS Pharma Biomedical were cultured in a 5% CO2 atmosphere at 37°C in RPMI-1640 medium (WAKO Pure Chemical Industries, Ltd., Osaka, Japan) containing 10% fetal calf serum supplemented with 100units/ml each of penicillin and streptomycin (WAKO Pure Chemical Industries, Ltd.). HWP-c cells obtained from PromoCell (PromoCell GmbH, Heidelberg, Germany) were cultured in a 5% CO2 atmosphere at 37°C in Preadipocyte Growth Medium (PromoCell GmbH). After reaching confluency, cells were cultured for 72h in Preadipocyte Differentiation Medium (PromoCell GmbH). Next, cells were cultured in Adipocyte Nutrition Medium (PromoCell GmbH) until they matured into adipocytes.
THP-1 cells were treated with ultra-pure Escherichia coli LPS (100pg/ml, 10ng/ml, or 1g/ml) (InvivoGen Corporation, San Diego, CA, USA) in a 5% CO2 atmosphere at 37C for 3h, washed with PBS (−) (WAKO Pure Chemical Industries, Ltd.), and resuspended in RPMI-1640 medium (WAKO Pure Chemical Industries, Ltd.). THP- 1 cells and adipocytes were co-cultured in a 5% CO2 atmosphere at 37C in RPMI-1640 medium (WAKO Pure Chemical Industries, Ltd.) using a cell culture insert with a 0.4mm porous membrane (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) to separate the upper and lower chambers. THP-1 cells were cultured in the upper chamber at 2 × 105 cells/ml, and adipocytes were cultured in the lower chamber at 2 × 105 cells/ml. THP-1 cells and adipocytes were collected on day 5 after the initiation of co-culture.
Total RNA from THP-1 cells and adipocytes was extracted using TRIzol® Reagent (Invitrogen Corporation, Carlsbad, CA, USA), according to the manufacturer’s protocol. RNA was quantified by absorbance at 260nm. cDNA was synthesized using reverse transcriptase with Oligo(dT)20 (TOYOBO Co, Ltd., Osaka, Japan).
Real-time PCR was performed using SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories, Inc. Hercules, CA, USA) on a MiniOpticon (Bio-Rad Laboratories, Inc.). The primers used have been previously described [26]. PCR conditions were set at 95°C for 3min, followed by 40 cycles of 95°C for 10s and 60°C for 30s. Relative quantification was performed by normalizing target expression to the housekeeping gene β-actin. Data are expressed as change (n-fold) in mRNA expression compared with that of THP- 1 cells incubated without LPS or adipocytes before co-culture.
The gene expression in LPS-pretreated (10ng/ml) THP-1 cells and adipocytes on day 5 of co-culture was analyzed using the fibrous DNA microarray Genopal® (Mitsubishi Rayon, Tokyo, Japan). Genopal®, which consists of gel-filled plastic fibers, has high reproducibility and high sensitivity [27]. DNA oligonucleotide probes were used to detect for 208 genes related to lipid and sugar metabolism. Data are expressed as change (n-fold) in gene expression compared with that of THP-1 cells or adipocytes before co-culture.
Data from the four experiments are presented as mean ± standard deviation. Data analysis was performed using a twotailed Student’s t test. Statistical significance between groups was considered at P < 0.05.
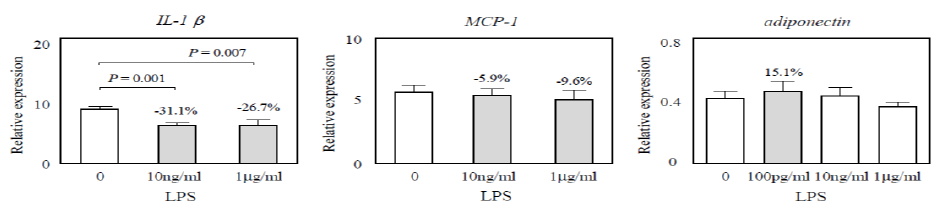
We investigated changes in the mRNA expression of inflammatory factor IL-1βand chemotactic factor MCP-1 in adipocytes on day 5 of co-culture. The mRNA expression of IL-1β and MCP-1 in adipocytes increased following co-culture with THP-1 cells. The increased mRNA expression of IL-1 β in adipocytes after co-culture was suppressed by 31.1% and 26.7% following interaction with THP-1 cells pretreated with 10ng/ml and 1g/ml LPS, respectively (P = 0.001, P = 0.007) (Figure 1). The increased mRNA expression of MCP-1 in adipocytes after co-culture was slightly suppressed by 5.9% and 9.6% following interaction with THP-1 cells pretreated with 10ng/ml and 1g/ml LPS, respectively (Figure 1).
Plasma levels of adiponectin were known to be reduced in obese patients [28]. Therefore, we investigated changes in the mRNA expression of adiponectin in adipocytes on day 5 of co-culture. The mRNA expression of adiponectin in adipocytes decreased following co-culture with THP-1 cells. The decreased mRNA expression of adiponectin in adipocytes after co-culture increased by 15.1% following interaction with THP-1 cells pretreated with 100pg/ml LPS (Figure 1). These results suggested that the mRNA expression of inflammatory factor and chemotactic factor in adipocytes after co-culture is regulated by LPS-pretreated monocytes and that an increase in mRNA expression of adiponectin is due to the antiinflammatory effect of low-dose LPS-pretreated monocytes.
Figure 1: mRNA expression in adipocytes. The mRNA expression of IL-1β, MCP-1>, and adiponectin in adipocytes on day 5 of co-culture with LPS-pretreated THP-1 cells was analyzed using quantitative real-time PCR. Relative quantification was performed by normalization to the value of the housekeeping gene β-actin. Data are expressed as change (n-fold) in mRNA expression compared with that of adipocytes before co-culture.
We investigated changes in the mRNA expression of inflammatory factor IL-1β and chemotactic factor MCP-1 in THP-1 cells on day 5 of co-culture. The mRNA expression of IL- 1βand MCP-1 in THP-1 cells increased following co-culture with adipocytes. The increased mRNA expression of IL-1βin THP-1 cells after co-culture was slightly suppressed by 6.4% and 6.0% with LPS pretreatment of 10 ng/ml and 1g/ml, respectively (Figure 2). In addition, the increased mRNA expression of MCP-1 in THP-1 cells after co-culture was significantly suppressed by 63.2% and 63.0% with LPS pretreatment of 10ng/ml and 1μg/ml, respectively (P = 0.0017, P = 0.0018) (Figure 2). These results suggested that the mRNA expression of chemotactic factor in THP-1 cells after coculture is more strongly regulated by LPS-pretreatment than that in adipocytes.
Figure 2:mRNA expression in monocytes. The mRNA expression of IL-1 β and MCP-1 in LPS-pretreated THP-1 cells on day 5 of co-culture with adipocytes was analyzed using quantitative real-time PCR. Relative quantification was performed by normalization to the value of the house keeping gene β-actin. Data are expressed as change (n-fold) in mRNA expression compared with that of THP- 1 cells incubated without LPS before co-culture.

We investigated changes in the expression of 208 genes in adipocytes and THP-1 cells on day 5 of co-culture using a semiquantitative fibrous DNA microarray. It was demonstrated that the increased mRNA expression of CD14, CCL5, and CD86 in adipocytes was suppressed by 37.7%, 16.3%, and 68.4%, respectively, following interaction with LPS-pretreated (10ng/ml) THP-1 cells (Figure 3). In addition, the increased mRNA expression of CD14 and IFNA5 in THP-1 cells after co-culture with adipocytes was demonstrated to be suppressed by 14.2% and 12.0% with the LPS pretreatment of 10 ng/ml, respectively (Figure 4).
Figure 3:Gene expression in adipocytes using a DNA microarray. Gene expression in adipocytes was analyzed using a DNA microarray on day 5 of co-culture with LPSpretreated (10ng/ml) THP-1 cells. Data are expressed as change (n-fold) in gene expression compared with that of adipocytes before co-culture.

Figure 4:Gene expression in monocytes using a DNA microarray. Gene expression in LPS-pretreated (10 ng/ ml) THP-1 cells was analyzed using a DNA microarray on day 5 of co-culture with adipocytes. Data are expressed as change (n-fold) in gene expression compared with that of without LPS treatment before co-culture.

Our previous reports have demonstrated that the increased mRNA expression of inflammatory factors, IL-1β, IL-8, VEGF-A, TNF- α in human monocytes by humoral factor-mediated interactions with cancer cells was suppressed by low-dose LPS pretreatment. These results suggested that low-dose LPS-pretreated human monocytes regulate the production of inflammatory cytokines in tumor tissues [16-18]. In this study, we demonstrated that the increased mRNA expression of the inflammatory factor IL-1β in human adipocytes was significantly suppressed by interaction with LPS-pretreated human monocytes (Figure 1). In addition, the decreased mRNA expression of the anti-inflammatory factor adiponectin in human adipocytes was increased by interaction with LPS-pretreated human monocytes (Figure 1). These results suggested that LPS-pretreated human monocytes may have anti-inflammatory effect in adipose tissues as well as tumor tissues. Therefore, LPS-treated human monocytes may regulate inflammatory reactions. It has been shown that inflammatory changes observed in adipose tissues with visceral obesity contribute to the development of metabolic syndrome [19-22]. Suppressing expression of inflammatory factor using LPS may thus help to prevent the occurrence of metabolic syndrome. It was reported that the chemotactic factor MCP-1 increased in adipose tissues with obesity and that infiltration of macrophages into adipose tissues was performed via the MCP-1 receptor,C-C chemokine receptor-2 [29-31]. MCP-1 has been shown to play an important role in the accumulation of macrophages in adipose tissues with obesity. Our data indicated that the mRNA expression of MCP-1 in human adipocytes increased spontaneously by interactions with human monocytes, and that this increase was slightly suppressed by interaction with LPS-pretreated human monocytes. In addition, the increased mRNA expression of MCP-1 in human monocytes by interaction with human adipocytes was significantly suppressed by LPS-pretreatment. The regulation of expression of MCP-1 by LPS may inhibit infiltration of macrophages and subsequently decrease accumulation of macrophages in adipose tissues.
Recent reports have shown that macrophages infiltrated the arterial wall and form foam cells, and that they induce inflammatory changes in tissues by secreting inflammatory cytokines [9-11]. It has been suggested that chronic inflammation in obesity affects the occurrence of diabetes [12,13]. The regulation of MCP-1 expression by LPS may help to prevent the occurrence of diabetes. Thus, LPStreated human monocytes may be beneficial for the prevention of diseases caused by chronic inflammation.
DNA microarray analysis findings demonstrated that the increased mRNA expressions of CD14, CCL5, and CD86 in human adipocytes were suppressed by interaction with LPS (10ng/ml). Pretreated human monocytes. Also, it was demonstrated that the increased mRNA expressions of CD14 and IFNA5 in human monocytes following interaction with human adipocytes were demonstrated to be suppressed by LPS (10ng/ml) pretreatment. CD14 is known to be a receptor of LPS. The molecular responses of human monocytes following interaction with human adipocytes may be tolerated by LPS-pretreatment. As previously reported, DNA microarray analysis findings demonstrated that the increased mRNA expression of CD86 in human monocytes following interaction with hepatic cancer cells was suppressed by LPS-pretreatment [18]. CD86, a T-cell co-stimulatory molecule, was shown to contribute to T-cell suppression [32]. Therefore, it is suggested that LPS-treated human monocytes regulate inflammation response through T-cell regulation in adipose tissues as well as tumor tissues.


