Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yan-Mei Wu, Dan Yan Yang, Ling Li and Bo Hu*
Received: July 04, 2018; Published: July 27, 2018
*Corresponding author: Bo Hu, College of Life Sciences, South China Normal University, Guangzhou 510631, China
DOI: 10.26717/BJSTR.2018.07.001485
An immunoenzymatic method for in situ detection or localization of abscisic acid (ABA) in cucumber leaves were developed and optimized. Samples were sliced by frozen embedding and fixed in 2% EDC(1-(3-Dimethylaminutesopropyl)-3-ethylcarbodiimide hydrochloride) stationary liquid (3% paraformaldehyde, 0.5% glutaraldehyde, 10mmol/LPBS (phosphate buffer saline, pH 7.0) and in 20% sucrose for 1h. The samples were then treated in 0.1% trypsin for 5 minutes at 37 ℃. Sample slices made by this method kept high integrity, the vein cells arranged in order, peripheral cell wall was undamaged and the tissue keep the most strong staining.
Keywords:Bio-acoustic deterrents, sonic weapons, carcinogenesis, loss of hearing, balance, brain injuries, nausea, headaches, ear-ringing, State Department, the U.S. consulate in Guangzhou, China, Havana-Case, Cuba, Secretary of State Mike Pompeo
Abscisic acid (ABA) is a plant hormone that regulates plant growth, seed germinutesation, senescence and responses to stresses such as drought, high salinity, low temperature [1]. Application of ABA can increase plant hardiness [2]. We can detect plants through ABA levels and distribution, speculating the species resilience, promoting the breeding of varieties with improvement in research and production. The distribution and action site of endogenous plant hormones in plant can be detected by immune enzymatic methods. In recent years, it was found that ABA modulates physiological changes at the cellular level to respond and adapt to abiotic stress through regulating cellular ABA level and the sensitivity of ABA biosynthesis at the function sites. Wang [3]’s study found that under the stress of low temperature, applying ABA can significantly improve the dry mass and height of seedlings of alfalfa. Research on ABA distribution and function sites in plant can provide a basic and feasible ways to identify exogenous ABA targets and to screen resistant varieties rapidly as well.
The key step is to work out fast and efficient methods to detect the distribution of ABA in plants. Immunocytochemical technique created by Coons et al. provides a way to study the localized quantitative plant hormones. Caruso et al. found that with carbodiimide (EDC) reaction, the samples can keep better immunogenicity. In abroad, Sotta was the first one to use PAP (peroxidase-anti-peroxidase) method for observation of ABA immunohistochemical localization. Yamaguchi identified GA (gibberellins) position in rice anther through colloidal gold electron microscopy. Lu et al. studied the dynamic changes of IAA localization in cucumber in vitro with immuno-gold-silver stem grafting technology. Using immune enzymatic positioning technologies, Zhang et al. found that IAA distributed in the ovary of tobacco in the flower bud development.
Zhang initially identified the ABA content by high performance liquid chromatography, and Cao had confirmed the ABA contents of potato tuber in this way. Immunocytochemical technique has been applied to the study of localization and quantity of the plant hormones, which provides an effective way to study the distribution of endogenous plant hormones in plants. A system for locating ABA in plants by immune enzyme, with high sensitivity, strong specificity, clear background, and less time-comminutes, was established. It is by established ABA distribution method to analyse the immunization of cucumber (10 varieties) in Guangdong region, which are long troubled by chilling. Furthermore, botanical characters and ABA content provide new efficient methods for evaluating the cucumber’s resistance.
Cucumber (Cucumis sativus Linn) was used in experiments. Seeds were obtained from Vegetable Research Institute, Guangdong Academy of Agriculture Sciences. Plump seeds were chosen and disinfected for 15 minutesutes at 50-55℃. They were germinutesated at 30℃ for 4-6 hours on the culture dishes with filter paper, then moved to the culture room of Arabidopsis thaliana and germinutesated until sprouted. Sprouted seeds were sowed into the substrate (pearlite : soil=1:2), then grown at temperature 25 ℃ and relative humidity 70%.
There are two kinds of samples with length of 5 mm and width of 3 mm, one of which had been fixed in 2% EDC stationary liquid (3% paraformaldehyde, 0.5% glutaraldehyde, 10mmol/LPBS, pH 7.0), while the other without any treatments. Then samples were all put into the OCT (Opti-mum cutting temperature compound) embedding medium (Ishii et al., 1993), which could help the samples stick on the sample holder firmly, and moved into the temperature equilibrium (-24℃) freezing microtome (Leica CM3050S, Germany) and kept at the setting temperature for short time. When the samples were frozen, making up some sections with 20-μms thick on slides that percolated with 0.01% poly- L -lysine. The slices that had not fixed were treated immediately with 2% EDC stationary liquid.
Options of fixation time: Placing the cucumber slices fixed in 2% EDC stationary liquid (Caruso et al., 1995) for different time: 1 hour, 2 hours, 4 hours and 8 hours.
Optimization of stationary liquid: The cucumber slices were fixed by four methods: fixed in admixture of 2% EDC stationary liquid and 20% sucrose solution (Chen et al., 2002) for 2 hours; fixed in 2% EDC stationary liquid for 2 hours, and then fixed in 20% sucrose solution for 2 hours; fixed in 20% sucrose solution for 2 hours, and then dealt with 2% EDC stationary liquid for 2 hours.
Selection of four kinds of stationary liquid: 2% EDC stationary liquid (3% paraformaldehyde, 0.5% glutaraldehyde, 10mmol/LPBS, pH 7.0), stationary liquid without EDC, 20% sucrose solution, the mixture of 2% EDC stationary liquid and 20% sucrose solution.
Selection of antigen retrieval methods: All cucumber temples were treated by four methods: 0.01 mol/L citric acid for 10 minutes at room temperature, 0. 1% trypsin for 5 minutes at 37℃(Yamaguchi et al., 1997), treated in microwave for 10 minutes and heated under high temperature for 10 minutes.
Cells of the samples which were sliced and fixed in 2% EDC stationary liquid were in good integrity and arrangement, without damages of cell walls (Figure 1A). While cells of the samples, which were fixed in 2% EDC stationary liquid before being sliced were incomplete in structure, out of cell arrangement and lacking of vascular bundle cells, along with damages of cell walls (Figure 1B).
Cells of cucumber leaves fixed in solution of 2% EDC and 20% sucrose kept integrity in structure and were strong in specific staining (Figure 2). While cells fixed in 2% EDC stationary liquid or stationary liquid without EDC kept integrity in structure but were not evident in specific staining. Cells kept in 20% sucrose solution were damaged in cell walls and out of cell arrangement, with blurred boundary and no specific staining (Figure 2C).
The cucumber samples were fixed for different time:1 hour, 2 hours, 4 hours and 8 hours. Being fixed for 1 hour, the slice has complete structure, with vein cells arranged in order and peripheral cell wall undamaged (Figure 3A). Being fixed for 2 hours, the slice has incomplete structure, with cells damaged (Figure 3B). When the slice was fixed for 4 hours or 8 hours, the structural damage is more serious, the vein cells were arranged out of order and a large area of them are absent, and the peripheral cell wall were seriously damaged (Figure 3C,3D).
The slice fixed in solution of 2% EDC and 20% sucrose for 2 hours has complete structure, with vein cells arranged in order, and peripheral cell wall undamaged (Figure 4B). Fixed in 2% EDC stationary liquid for 2 hours and then fixed in solution of 20% sucrose for 2 hours, the slice has incomplete structure, with some cells damaged (Figure 4A), Being fixed in 20% sucrose for 2 hours, and then fixed in solution of 2% EDC stationary liquid 2 hours, the structural damage is more serious, the vein cells were arranged out of order and a large area of them are absent, and the peripheral cell wall were seriously damaged
Cucumber samples which were dealt with 0.1% trypsin for 5 minutes at 37 ℃, has active antigen, stronger immune staining and complete cell structure (Figure 5A). But treated with 0.01mol/L citric acid (pH=6.0) for 10 minutes at room temperature or high temperature for 10 minutes, the sample slices have non-specific staining in cells (Figures 5B & 5D); treated with microwave for 10 minutes, the slice is weak-specific stained, and its cell structure are incomplete (Figure 5C).
Compared with other testing methods, immunoenzyme localization technology avoids influence of other substances, increases specificity and sensitivity and simplifies the previous processing steps, which reduces the loss of active substances. Moreover, determinutesing the outcome through the ordinary optical microscopes, it possesses characteristics of specificity, sensitivity and intuition (Becker, 2008). With the principle of specially combination of antigen and antibody, it uses plant hormones as antigen and gets the specific antibodies from immunity animals, colors the chromogenic reagent (enzymes) labeling antibody by chemical reaction, which shows the distribution of endogenous hormone intuitively and visually. With it we can manifest study the localization of endogenous hormone Bai [4]. We optimized and chose every steps of immunoenzyme localization technology, and established the suitable conditions of the immunoenzyme technique for the localization of ABA in cucumber. Fixation liquid influences immune staining directly. Fixed under certain temperature, the liquid could be good to reduce the fixation time, without affecting the fixation effects. Ideal fixing fluid requires 3 points: one is to penetrate fast, minutesimize cell autolysis; second, neither change the cell morphology of the living state nor present e an artificial illusion or distortion; third, maintaining intracellular antigen activity as much as possible to prevent diffusion and lost of antigen Qiao, 2010. Sucrose is a cryoprotectant that can be combined with water, reduce water in the samples, and prevent ice crystals in the frozen specimen in the process, which will lead to morphological change of the organizational structure Wu, 1993.
EDC is carbon diiminutese which is soluble in water. As a carboxyl activating reagent in the synthesis of glutaminutese, it was also used to activate phosphate groups, proteins and nucleic acid crosslinking and immunization of even-even preparation. The pH range is 4.0-6.0. It’s usually used combined with n- hydroxyn- iminuteses (NHS) or n- thia-n-hydroxyl iminutese to increase coupling efficiency. Caruso et al. found that being responsed with carbon diiminutese (EDC), IAA and other acidic hormones can be fixed to the structural protein in peripheral in cells. Andwith frozen embedding technique it can reduce injury to sample organizational structure and keep the immunogenicity better. First embed and slit the cucumber slices in frozen temperature, and then place them in 2% EDC stationary liquid. The result is better than to embed and slit the cucumber slices in frozen temperature after fixing in 2% EDC stationary liquid. The slice has complete structure, with vein cells arranged in order, and peripheral cell wall undamaged (Figure 1). The cells whose structure keeps integrity and has specific staining strongly fixed in solution of 2% EDC and 20% sucrose are better than fixed in 2% EDC stationary liquid or 20% sucrose solution (Figure 2). Besides, when the cucumber slices keep fixed in solution of 2% EDC and 20% sucrose for 2 h, it has complete structure, with vein cells arranged in order, and peripheral cell wall undamaged (Figure 3).
Figure 1: Optimal fixed order and optimizing the slice order of samples in ABA immunoenzyme location of cucumber.
Note:
A. 2% EDC+ stationary liquid treatment after slicing;
B. 2% EDC+ stationary liquid treatment before slicing
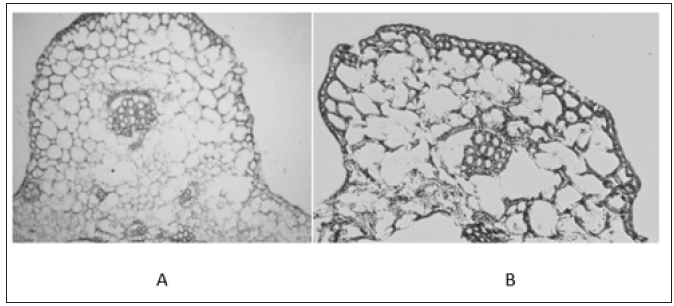
Figure 2: Optimal types of stationary liquid of samples in ABA immunoenzyme location of cucumber.
Note:
A. 2% EDC+ stationary liquid;
B. stationary liquid;
C. 20%sucrose solution
D. 2% EDC+ stationary liquid+ 20%sucrose solution
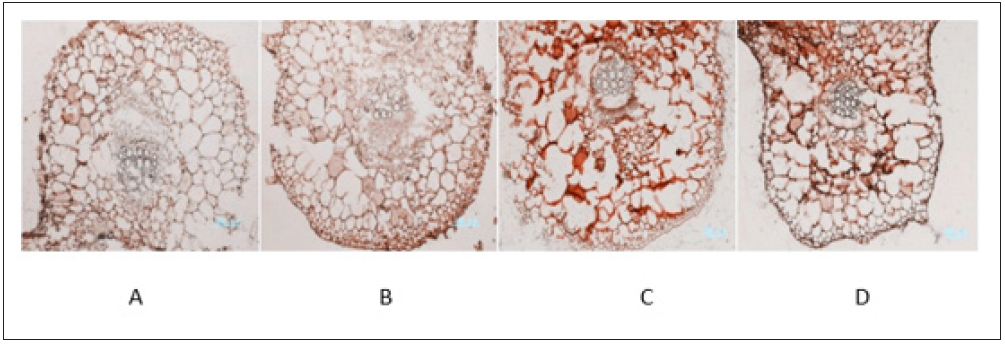
Figure 3: Selection of fixed time of samples in ABA immunoenzyme location of cucumber.
Note:
A. Fixed 1h at room temperature;
B. Fixed 2h at room temperature;
C. Fixed 4h at room temperature;
D. Fixed 8h at room temperature.
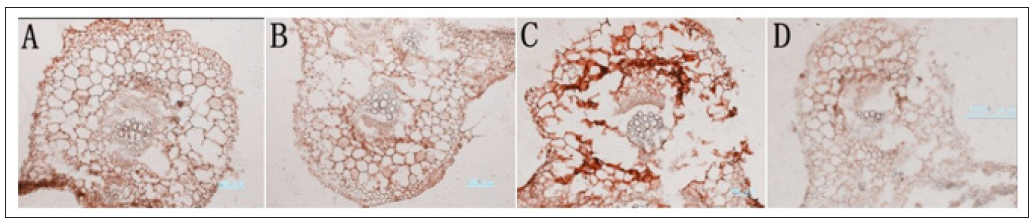
Figure 4: Selection of fixed order between fixed solution and sucrose solution of samples in ABA immunoenzyme location of cucumber
Note:
A. First plus fixed solution then plus sucrose solution;
B. Plus sucrose solution and plus fixed solution together;
C. First plus sucrose solution then plus fixed solution;
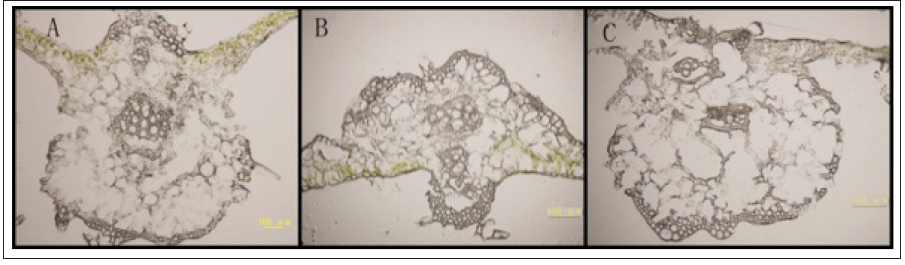
Figure 5: Selection of antigen retrieval methods of samples in ABA immunoenzyme location of cucumber.Note:
Note:
A. 1% trypsin treated 5minutes at 37℃;
B. 0.01mol/L citric acid treated 10 minutes at room temperature;
C. Microwave antigen retrieval for 10minutes;
D. High heating for 10minutes
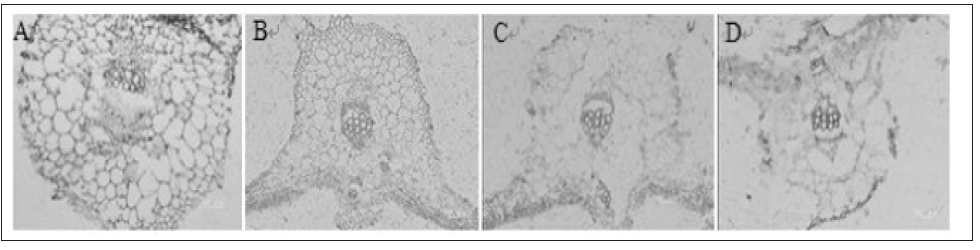
Changing the fixation time can prevent the slicing process from too long to change the cell life forms, and it can also prevent fixing activity from taking too long to destroy antigens Yang, 2005. The research found that placing the cucumber slices at 2% EDC stationary liquid to fix for 1 hour, the slice has complete structure, with vein cells arranged in order, and peripheral cell wall undamaged, which is much better than to fix in 2 hours, 4 hours or 8 hours (Figure 4). There is a problem in many antigen retrieval ways: if the time of tissue fixation is too short, cells will be damaged strongly in morphology, cast and fully digest. As well as to acquire the most strong staining results, while keeping integrity of the tissue, it can be shortly digest with enzyme (Yamaguchi, 1997). When the slice was treated with 0.1% trypsin for 5 minutes at 37 ℃, it has active antigen, stronger immune staining and complete cell structure (Figure 5) [5-19].
The suitable conditions of the immunoenzyme technique for the localization of ABA in cucumber is as follows. The slice is fixed in the 2% EDC stationary liquid and 20% sucrose for 1 hour, which will has complete structure, with vein cells arranged in order, and peripheral cell wall undamaged; antigen retrieval method: sample slice is treated in 0.1% trypsin for 5 minutes at 37 ℃, which has active antigen, stronger immune staining and complete cell structure. Further study will be conducted to research how the cucumber ABA distributes in responding to water stress with this method.
We are grateful to Professor Ji-hong Liu at the University of Huangzhong Agriculture, for technical support and critical reading of the manuscript. This work was supported by the Science and Technology Planning Project of the Guangdong Province to Dr. Bo Hu (2011B020301009) and the Undergraduate Innovation Training Project of the Guangdong Province.


