Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Basuki Supartono1, Errol Hutagalung2, Ismail2, Arief Boediono3, Toshiro Shirakawa4, Samsuridjal Djauzi5, Ahmad Aulia Yusuf6, Nuryati C. Siregar7, Jacub Pandelaki8, Adang Bachtiar9 and Katsumi Shigemura10
Received: July 11, 2018; Published: July 18, 2018
*Corresponding author: Basuki Supartono, Centre for Study of Cell and Tissue Engineering, University of Pembangunan Nasional Veteran, Jakarta
DOI: 10.26717/BJSTR.2018.07.001436
The knee joint cartilage often suffers from defect and it causes serious health problem. CD34+ stem cells have been studied to heal bone fracture however the cells has never been studied to repair cartilage. This study also focuses on the use of scaffold and growth factor affecting the healing of cartilage defect using CD34+ cells. This is the first study reporting hyaline cartilage regeneration on osteochondral defect with intra articular injection of human peripheral blood CD34+ (HPB CD34+) cellated on the trochlear region of Sprague Dawley (SD) rats. A total of 30 male SD rats were randomly divided into 3 groups; the control group received PBS, experimental group 1 received HPB CD34+ cells, and experimental group 2 received HPB CD34+cells, hyaluronic acid, and growth factors (TGF-ß1, IGF-1, FGF, fibronectin). Laboratory, radiology, macroscopic and microscopic evaluations were done on week 4th and 8th. At week 4th and 8th, both experimental groups showed the defects fully filled with hyaline cartilage but not in control group. In conclusion, human peripheral blood CD34+ stem cells can generates hyaline cartilage of osteochondral defects in a rat model.
Keywords: Stem Cell; CD34 + Cell; Hyaline Cartilage Regeneration; Osteochondral Defect; Knee Joint; Scaffold; Growth Factors
Abbrevations: SD: Sprague Dawley; MSCs: Mesenchymal Stem Cells; TGF: Transforming Growth Factor; IGF: Insulin-Like Growth Factor; FGF: Fibroblast Growth Factor; ACUC: Animal Care and Use Committee; HE: Hematoxylin and Eosin; DAB: Diamino Benzidine
Knee joint cartilage often suffers defects which cause pain, swelling, functional disturbances and disability, constituting a serious public health problem [1-3]. Damage is caused by aging, trauma, or other factors affecting cartilage. That process starts with subchondral pressure, deterioration of the cartilage surface, breakdown of cartilage layers and finally osteochondral defects on trochlear region of the knee joint [4-6]. Based on morphology and regeneration process, cartilage defects can be classified into superficial and deep defects. Superficial defects do not reach subchondral bone, and deep defects are those with tearing into the subchondral region [7,8]. Cartilage defects will trigger regeneration, but the result is not hyaline cartilage. In detail, defects will cause chondrocyte cells to proliferate, regenerate cartilage matrix and produced fibrous cartilage tissue [7]. Knee joint cartilage does not have blood vessels and therefore lacks the ability to regenerate [9-11]. Until recently, treatments of knee joint cartilage defect have not involved the production of hyaline cartilage [3,12,13].
Tissue engineering is promising for hyaline cartilage formation [4,14]. Chondrocytes are no longer used since they required a difficult and sophisticated technique and resulted in scar tissue [2,3,15,16]. So, we use tissue stem cells, which can be found in various tissues and organs, such as mesenchymal stem cells and hematopoietic stem cells [17,18]. Mesenchymal stem cells (MSCs) were first considered as a potential source but their application was limited by sample collection and cell characteristics [5,19]. The collection causes pain, morbidity, infection, and sepsis; in addition, the cell characteristics are affected by age. MSCs has to be cultured in advanced, before applied in therapy [11,20]. Alternatively, hematopoietic stem cells were easy to collect with minimal complications, provide high yield, and readily used in therapy regim [21]. Hematopoietic stem cells are progenitor cells which can be isolated from bone marrow and blood with either a direct or mobilization technique [21,22]. These stem cells are pluripotent and plastic, and can form non-hematopoietic cells such as fibroblast like cells, endothelial cells and osteoblast cells [23,24,25]. Hematopoietic stem cells can move into and regenerate damaged tissues [26].
A hematopoietic stem cell, CD34+, has been studied to heal bone fracture however the cells has never been studied to repair cartilage defect [21]. Our study will focus on the use of scaffold and growth factor affecting the healing of cartilage defect using CD 34+ hematopoietic stem cell. Hyaluronic acid was used as scaffold molecules as it was proven to improve the formation of chondrocyte and proteoglycan [14]. On the other hand the formation of cartilage was found to be affected by growth factors such as TGF-β1, IGF-1 and FGF which are also regulatory molecules in order to regulate cell behavior and increase chondrogenesis. [2,10,15,27]. Another important component is the availability of fibronectin in which will increase cell adhesion, migration and cell differentiation [28]. The objective of this study was to investigate the efficacy of a suspension of human peripheral blood CD34+ stem cell, hyaluronic acid and transforming growth factor (TGF)-β1, insulin-like growth factor (IGF)-1, fibroblast growth factor (FGF), and fibronectin to regenerate hyaline cartilage in Sprague Dawley (SD) rats with osteochondral defects.
This study was conducted in three steps; pilot study, preparation of the suspensions, and final study. Pilot study was conducted in Bimana, Centre for Non human Primate Agricultural University of Bogor. Preparation of suspension and final study were conducted in University of Indonesia. All procedures of pilot study had been approved by Animal Care and Use Committee (ACUC) Bimana the Ethical Committee Centre for Non-Human Primate, Bogor Agricultural University Number R.03-12-IR. All procedures for preparation of suspension and final study involving human blood collection was sought from the Ethical Committee Medical Faculty University of Indonesia Number 812/PT02.FK.43/N/2012. All blood donors were volunteers who have been explained about the procedures and use of their blood samples in this study and have knowingly signed the inform consent. All donors had no history of hepatitis, HIV, malignancy or bone marrow disease and never had chemo- or radiotherapy. All blood samples were examined in laboratories for detection of HIV and Hepatitis B also for another blood examination such as liver and kidney function.
Figure 1: Normal Trochlear and Osteochondral Defects Model in Rat’s Knee Joint.
(A) Normal trochlear, (B) Osteochondral Defect Model: (B1) Superficial defect (black arrow) and (B2) Deep defect (red arrow). The superficial defect has no bleeding. The deep defect post micro-fracture demonstrates bleeding (yellow arrow). No bone destruction. Bar = 1 (A) and 1.1 (B) mm.
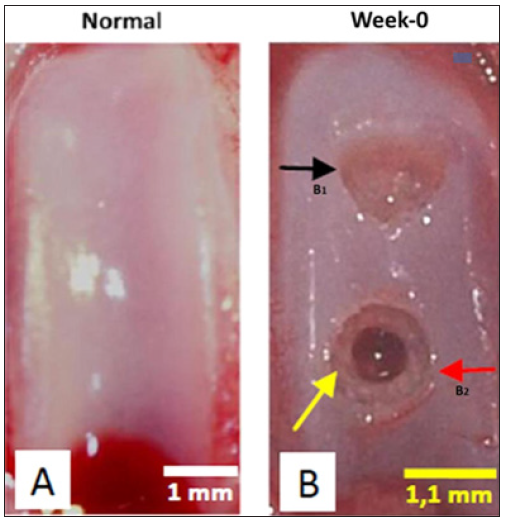
Thirty un-engineered naive rats were purchased from National Agency of Drug and Food Control, Ministry of Health of Indonesia, Jakarta. Rats at 11 weeks of age, 268 + 12-gram weight were housed and undergone adaptation with twelve-hour light/dark cycle and free access to food and water. Each rat was manipulated with two defects including superficial defect in proximal trochlear region and deep defect in distal trochlear region in the right knee joint at one time (Figure 1). Superficial defect was produced by drilling right trochlear with 1 mm stopper end which had 1.1 mm diameter (30% trochlear width). Deep defect was produced by doing a micro fracture of superficial defect with 0.8 mm K wire until it reached sub-chondral bone and bleeds. Then, the knee joints were irrigated with saline solution. After the surgery, rats were returned to their cages and could move freely. The rats were fed as needed and received paracetamol 300 mg/kg body weight and amoxicillin at 150 mg/kg bodyweight SC for 5 days.
Rats were randomized into 3 intervention groups; ten rats are mentioned as Control group receiving PBS. Ten rats in experimental Group 1 received human peripheral blood CD34+ stem cells. The remaining were in experimental Group 2 received human peripheral blood CD34+ stem cells plus hyaluronic acid and growth factor: TGF-ß1, IGF-1, FGF, and fibronectin. All interventions were injected directly to the operated knee joint in the day of operation. All groups were divided into 2 sub groups according to period of examination (4th week and 8th week after intervention) in laboratory, radiology, macroscopic and microscopic evaluations. Each group has 5 experimental rats.
Human peripheral blood were collected from 5 males, healthy donors at age between 18 - 35 years old, with an average body weight of 84 21 kg. All donors had no history of hepatitis, HIV, malignancy or bone marrow disease and never had chemo-or radiotherapy. All donors signed informed consent and undergone a preliminary blood examination. About 200 ml intravenous blood was collected from every donor. All procedures in taking and examination of blood samples were conducted in Al Fauzan General Hospital, Indonesia. All collected blood were isolated with Ficoll gradient centrifugation and selected to CD34+ using Mini Macs column system. CD34+ cells were counted and its viability was examined using Flowcytometry. Blood specimens were diluted with PBS + KCl solution, filtrated with Ficoll and centrifuged. Buffy coat layers were taken, washed and supernatant was removed until only MNC cells were available to be counted and viability checked. Afterward, MNC cells were washed and labeled by adding 300 μl buffer solution (autoMACS TM Rinsing Solution and MACS BSA Stock Solution, 20:1), FcR Blocking 100 μl, and CD 34+ micro-beads 100μl, and the suspension was incubated for 30 minutes at 2 – 8 0C.
Subsequently, 10 mL buffer solution was added to cell suspensions and centrifuged. The supernatant was removed and cells were re-suspended with 500 L buffer. Cells were separated with separator column. About 500 L of buffer was added into the column along with the cell suspension. The column was washed with 500 L buffer solution three times. CD34+ cells retained in the column were pushed with a syringe into a tube. Cell suspensions were centrifuged, the supernatant was removed, and cells were suspended. Interventional suspensions were subsequently prepared. Afterward each sample was identified by numbering.
Three suspensions were used:
a) PBS suspension,
b) 105 human peripheral blood CD34+ cells,
c) 105 human peripheral blood CD34+ cells with hyaluronic acid (Adant Dispo) 250 g/25 L, TGF-β1 (Biovision Cat No.4342-5) 1 g/ 5 L, IGF-1 (Sigma Cat. No. I3769) 1 g/5 L, FGF 1 g/ 5 L and fibronectin (Sigma Cat. No F2006) 2 g/ 10 L. All suspensions were diluted with 50 L PBS solution.
For laboratory evaluation, blood specimens were taken from the heart after rats were euthanized. One mL blood was taken into an EDTA tube and homogenized, and hematologic analyses were done with Sysmex (Kobe, Japan) K1000 hematology analyzer. Two mL blood were centrifuged in tubes at 3000 RPM for 15 minutes, and serum was taken for blood chemistry evaluation (SGOT, SGPT, Ureum and Creatinin serum level) and spectro photometry (TC84plus) [29].
Each rat was examined radiologically and macroscopically in both knees right after osteochondral defect created and it was documented as control. In the week 4 and 8, rats were then euthanized with pentobarbital intraperitoneally. We observed the defect model by radiography. We performed antero-posterior (AP) and Lateral (L) knee joint X-ray then evaluated using radiologic score. Based on Kellgern-Lawrence modification, we divided the radiologic score into 4 categories: signs of osteophytes (No=0; Yes=1), signs of sub-chondral schlerosis (No=0; Yes, unclear=1; Yes, clear=2; yes, clear with femoral, tibial or patelar cyst=3), joint gap (Normal=0; Narrow=1; No gap=2), joint deformity (None=0; Unsperical femoral condyle=1; clearly destruction and deformity=2)28. Radiography was followed by general morphological evaluation to observe the repair of the defect of the manipulated knee, then documented using Olympus SLR E-620 [30]. The macroscopic evaluations used a macroscopic score. Based on Nishimori modification, we divided macroscopic score into 4 categories: Sign of infections (No=0; Yes=1), Sign of allergy (No=0; Yes=1), Defect surface (Covered with white tissue, flat and smooth surface/normal=0; Covered with white tissue, un-flat surface=1; Covered by transparent layer=2; Not covered=3), Defect margin (Cannot differentiate with healthy tissue=0; Difficult to differentiate=1; Easily to differentiate=2; Clearly marked=3) [31].
Specimens at 4th and 8th week were fixed with 4 % paraformaldehyde and 70 % alcohol alternately for 24 hours at 4 oC, decalcified with Plank & Rychlo for 10 days, dehydrated with alcohol then encased in paraffin and sectioned in 5um thick slices and stained with Hematoxylin and Eosin (HE) and Safranin O/ Fast Green [32]. The specimens used for immune histo chemistry and collagen examinations were deparaffinized, rehydrated with running water, and washed in aqua, incubated in a sequence with protein blocker (BIOCARE’s Background Sniper solution), antibodies (TGF-β1, IGF-1, FGF, and fibronectin) in ratio 1:150, PBS and BIOCARE’s Trekkie Universal Link solution, and PBS and BIOCARE’s TrekAvidin-HRP. After incubations, the specimens were washed with PBS and diamino benzidine (DAB) solution, and then counterstained with hematoxylin. For the last procedures, the specimens were washed again with aqua, dehydrated, cleared and mounted.
Qualitative and quantitative examinations were performed using a light microscope and microphotography tools and evaluated using Pineda microscopic score based on Safranin O/Fast Green staining. The microscopic score was divided into 4 categories: Filling of defect in percentage (125%=1; 100%=0; 75%=1; 50%=2; 25%=3; 0%=4); Reconstruction of osteochondral junction (Yes=0; Almost=1; Not close=2), Matrix staining (Normal=0; Reduced=1; Significantly reduced=2; Faint=3; None=4); Cell morphology (Normal=0; Mostly hyaline and fibro cartilage=1; Mostly fibro cartilage=2; Some fibro cartilage but most non-chondrocytic cells=3; non-chonrocytic cells only=4) [33].
Data were descriptively and comparatively analyzed by SPSS version 17.0 and expressed as mean + SD. The comparative analysis from macroscopic, microscopic and radiologic scores were evaluated using Manova Test. P < 0,05 was considered statistically significant [34].
Radiological and Macroscopic Evaluation
No rats were sick, disable, or died during the experiment and wounds healed well. Rats exhibited normal activity such as walking, climbing, eating and drinking. During the study, all rats gained body weight in the week 8. Statistically, there was no significantly different between control and experimental group 1 and 2 in weight gain (Anova Repeated Measure Test, p = 0.139). Superficial and deep defects were successfully made in the right trochlear without causing bone destruction (Figure 1). The trochlear sizes of control rats and experimental groups 1 and 2 were 22.8 3.3 mm, 23.1 4.2 mm and 21.4 3.4 mm, respectively. There was no significant difference in the trochlear size (Anova test, p = 0.567, CI 95%). Defect size was 0.91 mm2 or approximately 4.2 0.57 % of the control group’s trochlear size or 4.2 0.8 % of experimental Group 1’s trochlear size and 4.5 0.73% of experimental Group 2’s trochlear size. Defect size comparison and trochlear size were not statistically significant (Anova test, p = 0.585, CI 95%).
The results of donor blood examination were Hb level of 14 1 g/dL, leukocyte count of 9.0 1.5 x 103/mm3 and platelet count of 299 74 x 103/ L. The amount of CD34+ cells varied between 1.15 x 105 - 25 x 105 cells with an average of 9 9 x 105 cells, with 96 2 % viability. After being combined, total cell counts were 11.3 x 105 cells with 95.3 % viability.
Hematology results of control and experimental Group 1 and 2 were not significantly different, except for the erythrocyte level. (Anova test, p= 0.16 (Hb), p= 0.00 (erythrocyte), p= 0.09 (platelets) and p= 0.56 (leukocytes), respectively). Same situation found in blood chemistry examination, that the SGOT and ureum level of rats in the control group and experimental Group 1 and 2 were not significantly different (Anova Test, p = 0.373 (SGOT) and p = 0.286 (Ureum), except for SGPT and creatinine level (p = 0.000 (SGPT) and p = 0.029 (creatinine)).
Note: osteophyte (none = 0, yes = 1), sclerosis (none = 0, unclear = 1, clear = 2, cyst = 3), joint gap (normal = 0, diminished = 1, no gap = 2), deformity (none = 0, non-spheric = 1, deformity = 2).
Knee joint radiological examination of rats on week 4 showed subchondral sclerosis of the knee joint in all groups. This condition persisted until week 8 in all groups. Regarding to radiological scores of the control group, experimental Group 1 and 2 were not significantly different (Manova test, p = 0.074). There was no significant difference of radiologic score on week 4 and 8 in all groups (Manova test; p= 0.200 (radiological); p = 0.446; p= 0.239 as shown in Table 1. Radiological score results for each variable on week 4 and 8 are shown in Table 2. On macroscopic evaluation, there were no signs of infection or allergy in the control and experimental groups for either superficial or deep defects on week 4 and 8. Macroscopic variables that changed were on the surface and margins of the defects as shown in Figure 2. The control group showed almost similar manifestation on week 4 and 8 in both defects which their margins were easily differentiated from normal surroundings and its surfaces were depressed but covered by whitish layer of cartilage. The experimental group 1 showed good alteration in defect margins and surfaces on week 4 and getting better on week 8.
Figure 2: Macroscopic Images of Superficial and Deep Defect after Intervention. Macroscopic images of superficial and deep defects on Week 4 (A, B and C) and Week 8 (D, E and F). The ontrol group (A and D) showed similar manifestations on week 4 and 8. Margins of the defect were easily differentiated from normal surroundings on the superficial defect (black arrow) and deep defect (yellow arrow), and the surface of the superficial (black arrow) and deep (yellow arrow) defects were covered with a whitish layer. In the experimental group receiving CD34+ cells (B and E), the margins of the superficial (black arrow) and deep (yellow arrow) defects were difficult to differentiate from normal surroundings. The defect surface was covered with a whitish tissue layer which started to be finer and smoother on week 8. In the group receiving CD34+ cells, hyaluronic acid and growth factors (C and F), the margins of the superficial defect (black arrow) were hard to differentiate and increasingly difficult to differentiate on week 8. The surface of the superficial defect (yellow arrow) was covered with a whitish tissue layer on week 4 which started to be more fine and smooth on week 8. Bar = 1.1 mm.
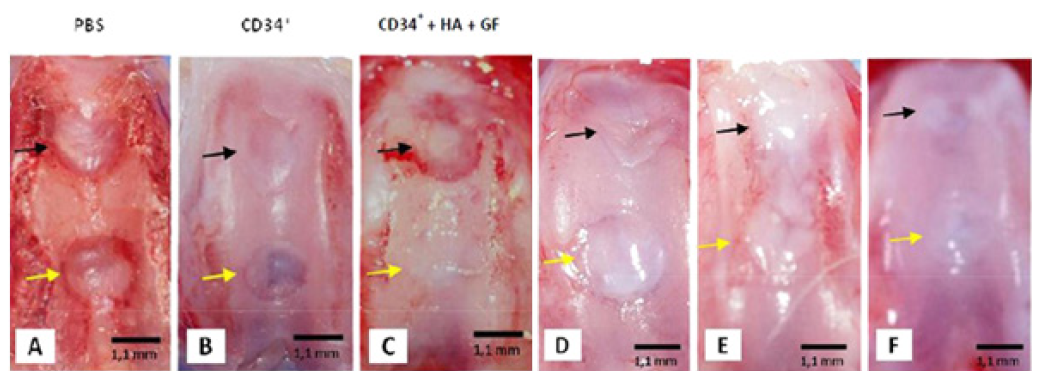
The defect margins were difficult to differentiate from surroundings and the defect surfaces were covered by whitish tissue layer which even started to be finer and smoother on week 8. The alteration of the defects was shown better in experimental group 2, which its defect margins were difficult to be marked from its normal surroundings on week 8 and its surfaces that covered by whitish tissue layer on week 4 altered to be smoother and finer features like normal tissue on week 8.Macroscopic scores for superficial defects and deep defects were significantly different calculated between the control group and experiment groups (Manova test; macroscopic superficial defect p = 0.000; macroscopic deep defect p = 0.000). Macroscopic scores between experimental Group 1 and 2 were not significantly different except for the macroscopic score of deep defects (Manova test; macroscopic superficial p = 1.000; macroscopic deep p = 0.023). According to the period of examination (4th and 8th week), there was not significantly different in macroscopic score for all groups in both superficial and deep defects (Manova test; p= 0.793 macroscopic superficial defect p= 0.507; macroscopic deep defect p = 0.350). Macroscopic scores of superficial and deep defects for each variable on week 4 and 8 were shown in Table 3.
Note: infection (none = 0, yes = 1), allergy (none = 0, yes = 1), surface (normal = 0, white tissue = 1, transparent = 2, not covered = 3), margins (cannot differentiate = 0, difficult to differentiate = 1, easily differentiate = 2, clearly marked margins = 3).
Microscopic changes occurred for all variables, such as defect filling percentage, reconstitution of osteochondral connection, matrix staining and cell morphology formation, as shown in Figures 3 & 4. There were no significant differences at 4th and 8th week between superficial defect and deep defect in microscopic evaluation, Hematoxylin Eosin, SAFO and Collagen II staining at 4th and 8th week in control group showed that the defects were filled 50% with fibrous tissue, uneven osteochondral, no feature of collagen and were colored green. Meanwhile both experimental groups at 4th week and 8th week showed that the defects fully filled with hyaline cartilage, collagen, even osteochondral and were red colored. Experimental group 2 were showing better with 100% filling defects and evidence of chondrocytes. Microscopic scores of superficial and deep defect for each variable on week 4 and 8 are shown in Table 4.
Figure 3: Microscopic Images of Superficial Defect on Week 4 and 8.
Histology results using Hematoxylin Eosin staining (A-F); SAFO staining (G-L) and Collagen II staining (M-R). Chondrocyte cells shown in Experimental group 1 and 2 (orange-red color) using SAFO staining. Collagen II shown in Experimental group 1 and 2 (brown color) using Collagen II staining. Chondrocyte Cell and Matrix didn’t appear in Control Group. All images are in 100 x magnification. N : normal tissue. R : Regeneration tissue. Bar : 0,1 mm.
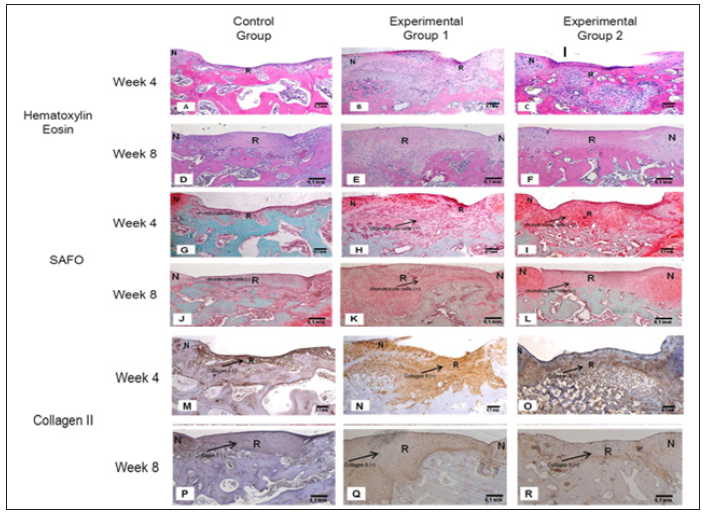
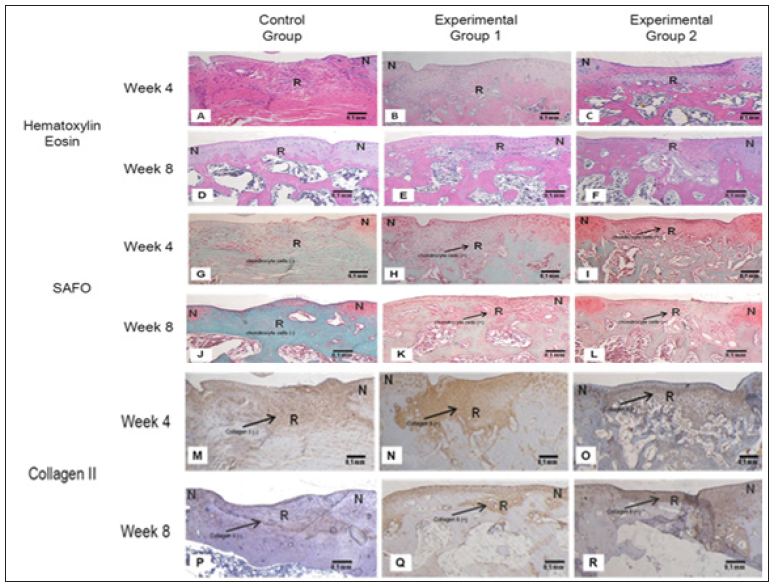
Figure 4: Microscopic images of Deep Defect on Week 4 and 8.
Histology results using Hematoxylin Eosin staining (A-F); SAFO staining (G-L) and Collagen II staining (M-R). Chondrocyte cells shown in Experimental group 1 and 2 (orange-red color) using SAFO staining. Collagen II shown in Experimental group 1 and 2 (brown color) using Collagen II staining. Chondrocyte Cell and Matrix didn’t appear in Control Group. All images are in 100 x magnification. N: normal tissue. R: Regeneration tissue. Bar : 0,1 mm.
Note: Percentage (125 % = 1, 100 % = 0, 75 % = 1, 50 % = 2, 25 % = 3, 0 % = 4), Reconstitution (yes = 0, almost = 1, not close= 2), Matrix (normal = 0, reduce = 1, Significantly reduced = 2, Faint = 3, none = 4), Cells (normal = 0, Mostly hyaline and fibrocartilage = 1, Mostly fibrocartilage = 2, Some fibrocartilage but most non-chondrocytic cells = 3, Non-chondrocytic cells only = 4).
Until now, there is still no satisfying treatment method in repairing cartilage damage that producing hyaline cartilage, both conservative and surgical therapy. Conservative therapy including drugs administration, physical therapy and life style changes, are until now questionable, also surgical methods which included fragment fixation, articular washing, and artificial articular implantation. With the development of molecular biology, stem cell technology and materials science and other related disciplines, tissue engineering technology offered great hope for the treatment of cartilage damage especially osteochondral defects. We used CD34+ hematopoietic stem cells because of its plasticity that can turn into osteogenic and vasculogenic lineage [24,25]. The amounts of CD34+ cells that were recovered varied due to variations in donor characteristics and the selection process. Donor characteristic variations included age, body weight, leukocyte cell count, and the amount of MNC cells. Factorial analysis was not performed due to limited samples. Further research into sample acquisition would lead to better correlations between donor characteristics and CD34+ stem cells amount in peripheral blood.
CD34+ stem cell viability was quite high at 96 2 % with variation from 92 to 98 %. The result was good because CD34+ cell selection was done immediately after samples were collected. In addition, the selection process followed recommended procedures. An automated selection process for CD34+ stem cells would be ideal to ensure the quantity and quality of the cells. We developed suspension of CD34+ cells isolated from peripheral human blood combined with growth factor including TGF ß1, IGF-1, FGF, and fibronectin and compared its efficacy with the CD34+ cells alone. Osteochondral defect on trochlear region right knee from rats are macroscopically and radiologically showing a good sign of healing process. Histology revealed that the knee with osteochondral defects were repaired and fully filled with hyaline cartilage and showing chondrocytes cells and collagen II matrix in the rat´s knee Administered with CD34+ cells and CD34+ with growth factors.
Therefore, this study demonstrated that human peripheral blood CD34+ stem cells in un-engineered naïve rats promoted cartilage regeneration. CD34+ stem cells were proven to trigger regeneration in osteochondral defects.
The study shows the adaptive nature of human peripheral blood CD34+ stem cells. CD34+ cells have plasticity and can form chondrocytes which further trigger hyaline cartilage regeneration. The differentiation mechanism by which hematopoietic stem cells developed into chondrocytes was not determined, but a trans-differentiation mechanism is suspected [35]. This study demonstrates a synergistic effect from the three engineered components of cartilage [14]. As for the effects of suspension injection on microscopic findings, the score of superficial and deep defects in rats receiving a mixed suspension of CD 34+cells, hyaluronic acid and growth factors were better than rats receiving only CD34+cells, even though the difference was not statistically significant. Microscopic scores were based on SAFO staining results, which had four variables: defects filling, osteochondral connection, color absorption, and cell formation. Cell morphology evaluation needs to be given higher points since chondrocyte cells produce all the cartilage components. Beside SAFO/Fast Green staining, collagen II staining is also essential because this staining ensures the formation of collagen II matrix, which is the main characteristic of hyaline cartilage.
Alternatively, the doses of hyaluronic acid and growth factors used may not have been optimal to regenerate two defects in one knee. In the other hand we could see in the radiological examination that the cartilage defect created subchondral sclerosis. This is an early sign of osteochondral defect. This sign still persisted at 8 weeks with pure CD34+ stem cells or with a CD34+ suspension with hyaluronic acid and growth factors. This result is similar to the study conducted by Matsumoto who used CD34+ stem cells for rat femoral fracture healing [25]. Saw et al. reported that improvement in bone sclerosis was seen at 2 years after mesenchymal stem cells were given to heal osteochondral defects [36]. It concluded that sclerosis was a sign that the defect had not been completely healed. Regarding the effects of micro-fracture on osteochondral defect regeneration, the results showed that micro-fracture does not have a better regeneration effect. Microscopic findings and scores for deep defects were not better than for superficial defects. This is similar to other reports that micro-fracture evaluation at 12 months only showed fibrous cartilage [36].
In other words, stem cells and growth factors have no effect in increasing regeneration of micro-fracture. Micro-fractures also resulted in a non-flat defect surface [37]. Therefore, the need for micro-fracture for cartilage defect treatment should be reconsidered. Our study showed that longer periods of observation time did not improve regeneration of osteochondral defects in this model. The optimal time for osteochondral defect regeneration remains undetermined. There is no standard observation time-frame. Some researchers observe osteochondral defect regeneration for over 6 months [37]. Those studies modeled one defect on one knee joint. No study of osteochondral defect regeneration with our defect model has been reported to our knowledge. Further studies are necessary to optimize the healing time of osteochondral defects. In addition, every administration of new therapy should consider toxic reaction possibility. Our study has shown that no rats had any toxic or rejection reaction after CD34+ stem cell suspension injection. It provides evidence that human peripheral blood CD34+ stem cells can be given to un-engineered naïve rats without immunosuppressant drugs.
Others researchers, Terayama, Matsumoto used athymic rat for their bone regeneration studies [24,25]. Ferreras used naive rats with immunosuppressant drugs for his neuro regenerative research [38]. Our study provides evidence that stem cell study for cartilage regeneration can used naive rats. The result is promising for future stem cells studies because the experimental animals required no special or expensive treatments for immunosuppressive purpose for instance. The study was conducted for 8 weeks; therefore, no conclusions can be drawn about potential chronic reactions in the animals. A follow-up study with a longer time-course is needed to observe any chronic reaction. This study, however, is the first to show that human peripheral blood CD34+ stem cells did not cause rejection or toxic reactions in non-engineered SD rats. We would like to emphasize the implications and potential of further studies. First, human stem cell transplantation can be studied using nonengineered animals. This provides an opportunity for cost-effective pre-clinical research using human stem cells, especially CD34+ stem cells. Second, CD34+ stem cells have several advantages.
They can be easily isolated from peripheral blood using a safe, non-invasive and cheap method without the need for anesthesia, and can be directly applicable without culture. This opens up a vast opportunity, especially for cartilage tissue engineering and musculoskeletal tissue engineering. Third, this study opens up an opportunity for autologous stem cell transplantation for osteochondral defect treatment. The amount of peripheral blood CD34+ stem cells can be increased with mobilized drugs, and cells can be collected directly with an apheresis machine or by flow cytometry. This may be ideal for a safe, easy and comfortable therapy for osteochondral defects. Further studies were needed regarding therapeutic aspects of peripheral blood CD34+ stem cells in cartilage regeneration. This study will include CD34+ stem cells research and clinical research. CD34+ cell research is needed especially regarding donor characteristics and collection methods for CD34+ stem cells to find the safest, most cost effective methods with optimal results. Other studies regarding suspension volume and the concentration of CD34+ stem cells are also needed as well as studies on the effectiveness of scaffolds and growth factors.
Due to technical aspects and the expense of growth factors, other alternatives to be researched include isolation from donor peripheral blood. Further clinical research will address the effectiveness, safety and characteristics of the ideal osteochondral defect for peripheral blood CD34+ stem cell therapy. In addition, comparison with mesenchymal stem cells is also needed as an alternative therapy for osteochondral defects based on tissue engineering. Further study can also be enhanced with stem cell labeling, which is not provided in this study due to technical and financial problem. This would motivate the next researchers to complete this study.
This study demonstrated that intra articular injection of human peripheral blood CD34+ stem cells, hyaluronic acid and growth factor can generate hyaline cartilage of osteochondral defects in a rat model without compromising occurrence of adverse events. It might be further step to proceed to clinical study.
We thank Prof. Sarwono Waspadji for his invaluable help. We thank Drs. Ondri Dwi Sampurno, Ratih Rinendyaputri, and Dra. Rikrik A. Ilyas, and Diah Iskandriati for facility support and immunohistochemistry. We thank Katsumi Shigemura, Prita Kusumaningsih, Muhammad Faiz, Riyadi, Latifah, Andri Pramesyanti, Esti Cahyani Adiati for their invaluable help on this research.


