Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Aleksandra Mrela1and Zenon Pawlak2
Received: June 25, 2018; Published: July 13, 2018
*Corresponding author: Zenon Pawlak, Tribochemistry Consulting, Salt Lake City, UT 84117, USA
DOI: 10.26717/BJSTR.2018.06.001404
The wettability of the articular surface of cartilage depends on the condition of its surface-active phospholipid overlay, which is structured as a multi-bilayer. We examined the characteristics of this membrane surface entity in both its normal and degenerated conditions using a combination of atomic force microscopy, contact angle measurement, and friction test. The observations have led to the conclusions that the friction coefficient is significantly dependent on the hydrophobicity (wettability) of the surface of the tissue, thereby confirming the hypothesis tested in this paper
Keywords: Articular Cartilage; Phospholipid Bilayer; Wettability; Atomic Force Microscopy; Hydrophilic; Hydrophobic
Phospholipids are molecules present in various tissues and body fluids which are also named surfactants, substances which lower surface energy [1-3].The main phospholipid classes adsorbed onto the surface of cartilage surface-active phospholipids (SAL) were identified and quantified: phosphatidylcholine (41%), phosphatidyl ethanol amine (27%) and sphingomyelin (32%) were identified as the major components of the lipid bilayer coating a natural intact cartilage surface [4,5]. The human body naturally produces phospholipids. Phospholipids support most functions of organs, such as cardiovascular health, nerve health, liver function, digestion and, most importantly, certain phospholipids might act as boundary lubricants [1].
Figure 1: (a) An electron microscopy image of the articular cartilage surface of a human knee demonstrating the oligolamellar lining consisting of phospholipid bilayers [3]. The bar represents 50 nm and (b) Book cover “Articular cartilage: Lamellar-repulsive lubrication of natural joints” [1].
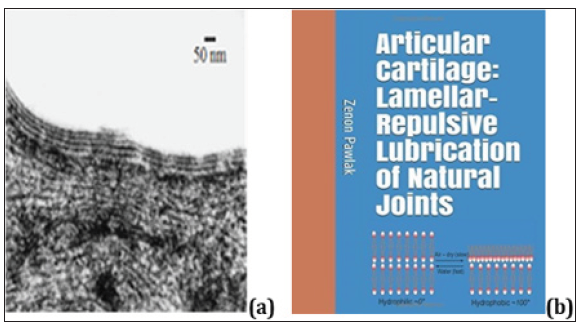
(Figure 1) (a) An electron microscopy image of the articular cartilage surface of a human knee demonstrating the oligo lamellar lining consisting of phospholipid bilayers [3]. The bar represents 50 nm and (b) Book cover “Articular cartilage: Lamellar-repulsive lubrication of natural joints” [1]. This smart surface characteristic creates a hydrophobic-hydrophilic balance resulting in a functional hydrophilic surface in the intact joint. One of the quantitative indicators of surface tribo chemical properties is wettability. This is measured as the contact angle between a drop of water and the reference surface, see (Figure 2a).
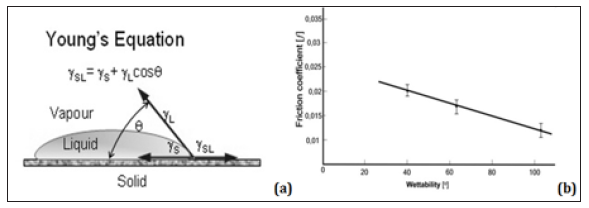
Figure 2: (a) Contact action for the wetting of a surface, where θ denote the contact angle, – the liquid free energy, – the solid free energy, – the solid/liquid interfacial free energy;(b) Coefficient of friction (ƒ) vs. wettability, ~ 0) of normal cartilage ( ~ 103)and gradually depleted ( ~ 65o and 39.1 and)cartilage surfaces measured in saline solution.
Wet ability characterizes the surface of various objects, which are generally defined as wettable (highly hydrophilic, ~ 0 to 45) or non wettable (highly hydrophobic, ~ 90 to 180) [2]. It is well known that polymers with hydrophobic entities (CH2- and CH3-) can be modified by oxygen-plasma-treatment to those carrying a hydrophilic group (-OH). This modification makes the surface hydrophilic with a small contact angle, which is effective in full aqueous lubrication [6, 7].
(Figure 2a) Contact action for the wetting of a surface, where θ denote the contact angle, – the liquid free energy, – the solid free energy, – the solid/liquid interfacial free energy; (b) Coefficient of friction (ƒ) vs. wettability, ~ 0) of normal cartilage ( ~ 103) and gradually depleted ( ~ 65o and 39.1 and)cartilage surfaces measured in saline solution. In this paper, we examine using some methods, such as AFM, together with surface wettability measurements, a relationship between wettability and friction. The wettability of cartilage depends on the number of PLs bilayers acting as a solid lubricant; this hypothesis was tested with normal and depleted cartilage samples. AFM imaging was used to examine the multi bilayer cartilage surface.
The articular cartilage specimens were collected from bovine knees aged 15-20 months. Osteochondral plugs, of 5 and 10 mm in diameter, were harvested from lateral and medial femoral condyles using a circular stainless steel cutter. The cartilage discs were cut into 3mm plugs with underlying bone. Two types of samples were tested: untreated bovine cartilage and bovine cartilage treated with a Folch reagent [8] (a 2:1 v/v mixture of chloroform and methanol), and a lipid-rinsing solution to remove the lipids from the surface of the cartilage. After preparation, the specimens were stored at 253 K in a 0.155 M NaCl (pH =6.9) solution and fully defrosted prior to testing. The cartilage discs were then glued to the disc and pin stainless steel surfaces, and friction tests were conducted in the universal buffer solution.
Tensiometer: The contact angle between the liquid and the tested cartilage was measured using a KSV CAM100 tensiometer. The contact angle measured was that between a droplet of a 0.15M saline solution and a given air-dry cartilage surface. The contact angle test performed on the normal, partial and completely depleted cartilage samples. Five tests were performed on each specimen and each set-up.
Friction test: The measurements were performed using a sliding pin-on-disc tribotester T-11 manufactured by the NIST Research, Radom, Poland. The tests were conducted at room temperature, at a speed of 1 mm/s during 5 minutes, and under a load of 15 N (1.2 MPa) which correspond to the physiological lubrication condition [9]. Prior to the friction tests, the lubricants were prepared using the Britton-Robinson buffer solution [10], and their pH values were measured. The friction coefficients measurements of cartilage/cartilage tribo pair were carried out over the pH range between 2.5 and 9.5.
Wetting of different surfaces (S) with a drop of saline: (a) when placed on surfaces with contact angles ( ) greater than 150°, (b) when placed on normal (an intact, air-dried) bovine articular cartilage) ( ,~100), and (c) when placed on an articular surface of a human hip diagnosed with osteoarthritis ( ,~40). (Figure 3a) The friction coefficient of (AC)/(AC) vs. wettability of surface (AC): (1) bovine and human cartilage surface 103° and 101° (2) knee 79.7° (3) unhealthy cartilage 65o, (4) knee 63° ,(5) degenerated hip 56.3°, (6) bovine cartilage surface partially depleted 63°, (7) partially depleted 53° and (8) completely depleted surface 35.1° [4, 11]. The wettability of cartilage surface was measured the under an air-dry condition at room temperature. (Figure 3b) The variation of wettability of AC vs the number of phospholipid bilayers. Note the typical inter lamellar aqueous spacing of 4.5 nm between bilayers [1]. Figure 3.3.5 in [1] represents the coefficient of friction vs. time for the (cartilage-cartilage) pair for normal and dilapidated surfaces. These friction tests thus confirm our hypothesis on the relation between the number of phospholipids bilayers (or wettability) and friction [12-16].
For normal (AC/AC) pairs the range of f = 0. 004 - 0.012, and in the ranges of f = 0.011- 0.09 unhealthy cartilage, and 0.015 - 0.024 for the depleted (AC-AC) pairs. This reveals the significantly higher friction coefficients between (AC-AC) pairs in the depleted conditions relative to those of the normal (AC-AC) pairs with intact contacting surfaces. The ratio of the coefficient of friction for the depleted (AC-AC) pair/normal (AC-AC) pair values after 5 min increased 2.3 and 3.0 times for the depleted (AC-AC) pairs. (Figure 2b) represents the plot of coefficient of friction (ƒ) vs hydrophobicity (wettability) for normal and dilapidated cartilage surface samples after 5 min of a test. The frictional characteristics of the treated cartilage were greatly increased (over 200%) relative to that of the untreated cartilage surface. Furthermore, it has been shown that the phospholipid on the surface of AC as bilayers is a lubricant and supports lubrication mechanism by lowering the friction of bio surfaces [17].
Figure 3: (a) The friction coefficient of (AC)/(AC) vs. wettability of surface (AC): (1) bovine and human cartilage surface 103° and 101° (2) knee 79.7° (3) unhealthy cartilage 65o, (4) knee 63° ,(5) degenerated hip 56.3°, (6) bovine cartilage surface partially depleted 63°, (7) partially depleted 53° and (8) completely depleted surface 35.1° [4, 11]. The wettability of cartilage surface was measured the under an air-dry condition at room temperature. (b) The variation of wettability of AC vs. the number of phospholipid bilayers. Note the typical interlamellar aqueous spacing of 4.5 nm between bilayers [1].
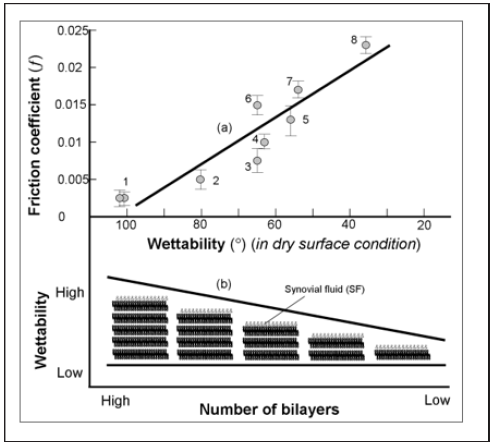
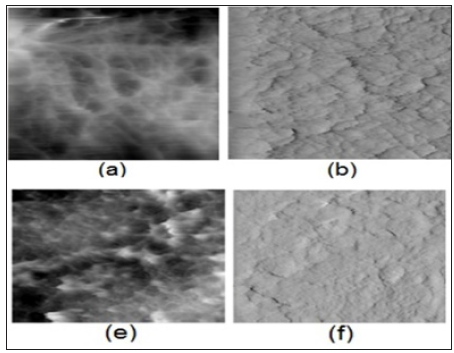
In (Figure 3a) (cartilage/cartilage) pairs were used to study the friction on cartilage surfaces under variable wettability. With the lipid-depleted cartilage samples and untreated normal samples, we were able to support observations made by other authors [4,16, 9].Our results demonstrated that wettability is an important factor for characterizing biological frictional surfaces. The friction coefficient vs. changes of wettability for natural joints with healthy and naturally degenerated articular surfaces [4] and bovine samples for partial and completely depleted cartilage samples, (Figure 3a & Figure 3b) shows changes in wettability of AC surface vs. numbers of bilayers. We interpret the increased friction coefficient values in terms of the number of bilayers available in the SAL [1,4,9]. Both friction and wettability show very similar behavior when the SAL thickness is varied [2,16,18]. The implication of this on cartilage has been observed during osteoarthritis where increased friction coefficient is measured due to gradual loss of the SAL [12,19,20]. Phospholipidic lamellar aggregates and bio macro molecules in SF may contribute to electrostatic hydration-repulsion during lubrication. The highly hydrated PL lamellar aggregates are expected to cover cartilage surfaces and support hydrophilic lamellarrepulsive hydration lubrication [20, 21].The results obtained from atomic force microscopy (AFM) imaging and characterization of the surface of normal intact and depleted articular cartilage specimens are presented in (Figure 4) Topographical (a, e) and deflection (b, f) 2-D images of articular cartilage surface (Frame size: 8 by 8 μm). Normal articular surface (a) and after a 21-min delipidization in chloroform/methanol (f). Wettability (a) 103o and (f) 39.1o.
Figure 4: Topographical (a, e) and deflection (b, f) 2-D images of articular cartilage surface (Frame size: 8 by 8μm). Normal articular surface (a) and after a 21-min delipidization in chloroform/methanol (f). Wettability (a) 103o and (f) 39.1o.
We have examined natural articular cartilage surface and shown the relation between surface wettability and friction. Tests were performed to measure the wettability of normal and degraded bio surfaces, while also measuring the coefficients of friction of contacting cartilage surfaces. The number of PLs bilayers influences both wettability and surface friction, which are fundamentally important factors in joint lubrication. The key to understanding the mechanism of joint lubrication lies in obtaining insight into the relationships between the structure and function of surface bilayers.


