Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Maryam Alhindi*, Hasan Muaddi, Mohammed Almosairy, Abdullah Alshebany and Malik Alabdulkader
Received: June 19, 2018; Published: June 28, 2018
*Corresponding author: Maryam Alhindi, Assistant Professor and ConsultantDepartment of Oral and Maxillofacial Surgery King Khalid University Hospital Faculty of Dentistry, King Saud University Riyadh Saudi Arabia, Saudi Arabia
DOI: 10.26717/BJSTR.2018.06.001315
Objective:To determine whether there is enough data to support prescribing NSAID is likelyhas negative effect on osseointergration or not.
Materials and Methods:The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement was used in this study. The clinical question in “PICO” format was: Do NSAIDs Inhibit the osseointegration of the dental implant? PubMed, MEDLINE, ScienceDirect and Cochrane Library was searched for articles published up until march 4, 2018.
Results: The search resulted in 371 articles. After selection according to the eligibility criteria, four studies fulfilled were included (Three RCT doubled blind and one retrospective cohort study) with a total of 217 patients analyzed, and 610 implants placed. Administration of a short course of systemic ibuprofen for post-operative pain management subsequent to implant placement may not have a significant effect on the marginal bone around dental implants in the early healing period.
Conclusion: The survival rate of implants in patients using NSAIDs in short term does not differ from the survival rate in healthy patients not using NSAIDs or using placebo. However, a greater number of prospective studies and clinical trials in the future is essential to support more solid conclusions.
Keywords: Dental Implants; Nonsteroidal Anti Inflammatory Drugs; Cyclooxygenase 2 Inhibitor; Meloxicam; Osseointegration; Bone Healing
Abbreviations: PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis; COX: Cyclo Oxygenase; DM: Diabetes Mellitus
During the last decade, dental implants are the first option to replace missing teeth. Developments in dental implant material, structural design, surface features, and surgical procedures made dental implants a secure and highly predictable procedure with a mean survival rate of 94.6 % and a mean success rate of 89.7 % after more than 10 years [1]. Highly survival rate of dental implant always depends on successful osseointergration after the placement; treatment outcome will deteriorate if there is any change of this biological process. Eventually, when an implant is restored by prosthetic and placed into function, bone remodeling becomes a serious aspect in responding to the functional requirements that placed on the implant restoration and supporting bone [2]. Osseointegration is a histologically characterize the intimate contact between the living bone and the surface of dental implant made of titanium at the light microscopic level of magnification [3,4]. This is the main target that can achieve a desired stability and strength to withstand and transfer the occlusal load during function without harming soft tissue [5,6].
After dental implant insertion bone will demonstrate sequence of initial healing contain inflammatory reaction, resorption of the bone at the site of insertion, growth factors releasing and attract the osteoprogenitor cells to the prepared site which is later on differentiate into osteoblast during the continuous process of bone remodeling [7]. Mild inflammatory response will promote healing of bone at the site [8]. Stability of dental implant must be achieved to enhance differentiation of osteoblasts and avoid fibrous tissue formation between implant surface and bone which may lead to nonintegration and implant failure [9]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used mostly in the dental clinics for management of acute and chronic inflammation and pain [10]. The main biologic effect of these anti-inflammatory drugs is the suppression of cyclooxygenase enzymes that is responsible to make Prostaglandins products [11]. There are three isoforms of cyclooxygenase: COX-1, COX- 2, and COX-3 [12].
COX-1 is constitutive that regulates normal cellular processes, and COX-2 is inducible and it induced by Inflammatory mediators and cytokines that produces by tissue injury, also it plays important physiological role on cardiovascular system. COX-3 is an alternative to COX-1 which is limited only in the central nervous system [13- 15]. COX-2 is an imperative for differentiation of mesenchymal stem cells into osteoblasts and also work as a main source of PGE2 that stimulate osteoblast to increase bone formation, bone mass, and strength. NSAIDs and COX-2 inhibitors directly affect the bone healing process via suppression of the COX-2 enzyme, which will decrease the amount of Prostaglandins, differentiation and activation of osteoblast in the early phases of bone healing [16]. This Review aim to determine if there is enough data to support that NSAIDs will have a positive effect on osseointergration of dental implant. Our rational is to support osseointergration of dental implant in patient by prescribing NSAIDs.
The substructure of the systematic review was based on the PRISMA statement [17]. The focused question according to the PICO schema is: “Do NSAIDs inhibit the osseointegration of the dental implant?
The databases were incorporated in the systematic search for relevant literature: PubMed,Medline, ScienceDirect and Cochrane Library. The following search terms were used: dental implants, nonsteroidal anti-inflammatory drugs or cyclooxygenase 2 inhibitor, meloxicam and osseointegration or bone healing, osteoprogenitor cell. Electronic search was complemented by an iterative hand-search in the reference lists of the already identified articles. The time period of the literature search will take about 3 months for collecting the data and 3 other months for writing the paper for publication. Endnote X7 was used for the electronic management of the literature.
During the first stage of study selection, the titles and abstracts will be screened and evaluated according to the following inclusion criteria: English language, retrospective and prospective clinical trials, observational studies, cross-sectional studies, cohort studies, and case series. During this procedure, the pre-selected publications will be evaluated according to the following exclusion criteria: Publications in languages other than English, in vitro studies, animal studies, case reports, published prior to 1990, diabetes mellitus (DM), hypertensive patient, Drug/alcohol dependency.
The studies were first assessed by title and abstract by two independent reviewers. A third reviewer was consulted to resolve any doubt about whether an article should be included or not. In the event that doubt remained, the article was included in the fulltext analysis. Finally, the complete texts were analyzed.
The quality and strength of evidence of the included studies was assessed by using a modified Downs and Black checklist. The checklist consisted of 27 different questions for the assessment of risk of bias and quality of the included studies, which asked for the reporting bias, external validity, internal validity and confounding (selection bias). The last question was adapted from the checklist used in another study assess the power of the study sample [18].
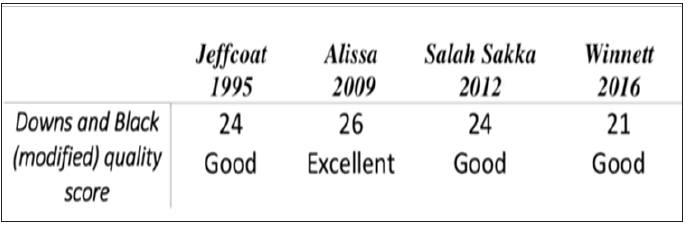
A flow chart of this systematic review, as recommended in the PRISMA statement, is given in the Figure 1. The search strategy initially yielded 371 articles. After the exclusion of duplicate articles, a total of 363 remained; the title and abstract of each of these were read. The inclusion and exclusion criteria were applied, resulting in the selection of four articles for full-text reading and data extraction. All studies were assessed for quality using the Downs and Black checklist [19,20]. The checklist is suitable for randomized and non-randomized studies, and covers study reporting, external validity, and internal validity. As in other reviews, the checklist was modified slightly. Item 27 was scored as 0 or 1 (rather than 0 to 5) giving each paper an overall score of 0 to 28. Three of our articles were having a good quality [21,23], where the last one was excellent [22].
The data presented in Table 1: Author and year; purpose; drug administration; study design; sample size; major finding were extracted from four studies. One of these four articles is retrospective cohort study and the remaining three articles are RCT doubled blind and all of them carried out in human. Over all this systematic review analyzed 217 patients (Total of 610 dental implants were evaluated in the 217 patients in retrospective study and clinical trials).The first study (Jeffcoat et al) investigating the influence of flurbiprofen on their patients who received dental implants. They reported no significant change in the bone height during the buried healing period of the implants [19,22] and [23] showed that administration of a short course of systemic ibuprofen 600 mg taken four times a day for post-operative pain management subsequent to implant placement may not have a significant effect on the marginal bone around dental implants in the early healing period [20,21]. In the study of Winnett et al evaluating 104 patients with 468 implants where the failure was 238 implants; out of these number 197 implants were due to failure of osseointegration (42%). They reported 60% with 273 implants had used NSAID preoperatively and they experienced 44% implant failure, where the non-NSAID had reported 38% failure rate 22 (Figure 2) and (Table 1).
Figure 2: Quality ratings of all studies (modified downs and black score); excellent quality (26-28), good quality (20-25), fair quality (15-19) and poor quality (less than 14).
We found four articles that evaluated the effects of NSAIDs on dental implant osseointegration (Table 1). Jeffcoat et al evaluated the effect of flurbiprofen (50 or 100 mg, twice daily for 3 months). They administered flurbiprofen for 3 months to their patients who received dental implants. Patients in the 100 mg flurbiprofen group experienced approximately half the bone loss of the 50 mg flurbiprofen in the placebo groups. However, after 1 year, the balance between bone loss and gain became stable in all of the groups. These results indicate that flurbiprofen at high doses may spare the bone around mandibular dental implants. No significant changes in bone height or mass were found between 6 and 12 months, indicating that bone loss stabilized even after flurbiprofen treatment was discontinued. Their trail provides preliminary data suggesting that systemic administration of the NSAID flurbiprofen may reduce bone loss around dental implants in the first year of service; however, the dose of NSAIDs used in this study were consistent with chronic use and not with those used for postoperative pain relief 19.
[22,23], evaluated the efficacy of a 1-week postoperative course of 600 mg ibuprofen taken 4 times daily on marginal bone level around dental implants. The primary outcome measured the change in marginal bone level around dental implants from the baseline (2 weeks post-placement) to the 3 and 6 months radiographic examinations. They found no statistical significant differences between different groups in the mean marginal bone level around dental implants at 3 and 6 months post placement. They concluded that administration of a short course of systemic ibuprofen for post-operative pain management subsequent to implant placement may not have a significant effect on the marginal bone around dental implants in the early healing period. But it is well documented that the evaluation of marginal bone loss using conventional radiographs has intrinsic limitations often related to the fact that conventional radiographs provide two-dimensional demonstration of a three-dimensional anatomy. Therefore, not only changes at a facial bone level may be unnoticed, but also sites that show an osseous change can be unobserved because of superimposition by unchanged bones. However, generalization of their findings should be made with caution because of the fairly small sample size of their patients recruited [20,21].
Winnett et al evaluated whether adverse biological events following oral implant placement may be associated with perioperative use of non-steroidal anti- inflammatory drugs (NSAIDs). They found that 238 implants failed out of 468, of which 197 (42%) were due to failure of osseointegration. Sixty of the patients with 273 implants used NSAID preoperatively and they experienced around 44% implant failures, where the non-NSAID cohort had experienced 38% implant failure, so no significant differences between the ibuprofen and non-ibuprofen groups in bone changes. The authors believed that the data are so clear to suggest that a relationship between the use of NSAIDs and failure of dental implants cannot be ruled out. This hypothesis is supported by a significant amount of physiological and pharmacological evidence that NSAIDs interfere with the healing of bone in cases of fracture or surgical treatment (e.g., joint prostheses). Also they suggested the need for further study-preferably prospective-to determine whether the observed data are due to the patient’s pre-existing limited bone healing capacity, or due to the direct effects of inhibition of prostaglandin synthesis on dental implant osseointegration caused by NSAIDs. They concluded that dental implant osseointegration may be affected negatively by an inhibitory effect of NSAIDs on bone healing in vulnerable patients [22].
The literature included to this review is associated with limited number of research studies performed in humans impeded a statistical analysis of the results (meta-analysis). Thus, the results offered to the reader are qualitative and not quantitative, allowing a certain degree of subjectivity in the conclusions. Further randomized clinical trials with longer follow-up period are needed because it remains unclear in what intensity the exposure to these medications is harmful to dental implant osseointegration. A greater number of prospective studies in the future are essential to support more solid conclusions.The scientific evidence for using NSAIDs pre and post dental implant placement in human is limited. The current literature review suggests that NSAIDs in conjunction with implant placement in long term gives a modest risk for implant loss. Analyzing the available studies, we concluded that dental implants are safe and predictable procedures for rehabilitation in patients under NSAIDs. The survival rate of implants in patients using NSAIDs in short term does not differ from the survival rate in healthy patients not using NSAIDs or using placebo.
The authors would like to thank Prof. Hesham Khalil, Maxillofacial Surgery Department and also College of Dentistry, King Saud University - Riyadh, Kingdom of Saudi Arabia, for his great support and guidance and encouragement.


