Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Khalid Al Nedawi*
Received: June 12, 2018; Published: June 19, 2018
*Corresponding author: Khalid Al Nedawi, Department of Nephrology, Canada
DOI: 10.26717/BJSTR.2018.05.001243
Keywords: Metabolism; Immunity; Proliferation; Exogenous; Retinoic Acid; Translocation; Retinoic Acid; Cancer; Genome; Receptors
Abbrevations: AR: Androgen Receptor; ER: Estrogen Receptor; EGFR: Epidermal Growth Factor Receptor; CRPC: Castration Resistant Prostate Cancer; PSA: Prostate Specific Antigen
Nuclear Receptors are a superfamily of transcription factors that exhibit multiplefunctions in health anddisease [1]. These receptors play essential roles in various physiological processes; including metabolism, immunity, developmental patterning and cell proliferation [2] typically nuclear receptors are activated bysteroid hormones, suchas estrogens, androgens, progesterone, and various other lipid-soluble signals, includingretinoic acid, oxysterols, and thyroid hormone. The ligands can cross the plasmamembrane anddirectly interact with the receptors in the cytoplasm, causing theirtranslocation to the nucleus [3]. Within the nucleus, the receptors bind DNA and activatespecific responsive genes. In cancer, nuclear receptors play amajor role in various typesof the disease, with roles ranging from oncogenic, tumor suppressor, or both, depending on the body organ they are expressed in [2]. Androgen receptor (AR) in prostate cancer and estrogen receptor (ER) in breast cancer are the most common nuclear receptors thatcontribute to theprogression of these cancers. The two receptors or their releasing factorsare targeted for the treatment ofthese cancers, by the hormonal therapies.
As part of the complexity of cancer it has been reported that manymembranereceptors could be translocated and detected in the nuclei of tumor cells, the phenomenonassociated with aggressive and therapy resistant tumors. One of the essential membranereceptors in the progression of various cancers is the epidermal growth factor receptor(EGFR), which is either overexpressedor mutated in most of the aggressive tumors. Ithas been reported that there is an alternative mode of EGFR signaling, in which activated EGFR undergoes nuclear translocalization, subsequently regulates geneexpression, andpotentially modulates cellular processes. This signalling route is distinct from thebettercharacterized,traditional EGFR pathway, which involves transduction of mitogenicsignals through theactivation of multiple signaling cascades. The signaling pathway ofnuclear EGFR is associated with increasedcell proliferation, nitric oxide synthesis andaccelerated G1/S cell cycle progression [4].
Several othermembrane receptors found toundergo nuclear translocation such as HER2, EBR3, Fibroblast growth factorreceptor 1and 2 (FGFR1 and 2), prolactin receptor, Interferon-γ receptor (INFγ), insulin receptor, and vascular endothelial growth factor receptor 2 (VEGFR2 or FlK1/KDR) [5-10] Nuclear translocated receptors have been shown to act as transcription factors [11-13] modulating transcription by either activation orrepression. Several mechanisms havebeen reported to explain the translocation of membrane receptors to the nucleus,including interaction with transport receptor importin β1, nuclear protein Nup358, and a host of players in endocytic internalization [14] Although the translocation of membranereceptors to the nucleusare well documented, the underlying mechanism of nucleartranslocation is poorly understood [5] In thisregard, the studies reported on thetranslocation of membrane receptors to the nucleus of the same cell. Theincreasinginterest of the scientific community in extracellular vesicles, which includes exosomesandmicrovesicles as a mode of intercellular communication in cancer drive a newunderstanding for their role inthe tumor microenvironment.
Particularly, the role of thesevesicles in the intercellular exchange of receptors [15,16] which led to questioning theirpossible role in shuttling these receptors to the nucleus of other cells. The recent finding [17] that extracellular vesicles could transport receptors directly to thenucleus of other cells that may not express the receptor, brings our understanding of thetumormicroenviron-ment to a completely new stage. The reported mechanism is novel, asall studies to date havereported only on the translocation of endogenous receptors fromthe cell membrane to the nucleus of thesame cell.The manuscript shows a new mechanism for the nuclear translocation of receptors suchas themutant form of EGFR the EGFRvIII, which has a truncation in the extracellulardomain that renders thereceptor active without the need for a ligand. The receptor isassociated with aggressive tumors inglioblastoma and other cancers such as prostate cancer [18,19].
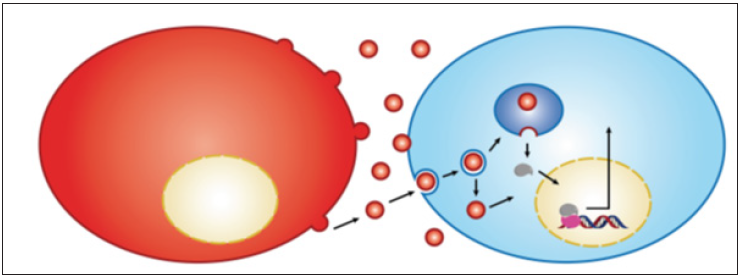
Although, the receptor has a weak signaling capacity, it is continuouslysignalling, which due to its impaired recycling through the endosomal pathway [20] makes it a potent oncogene. Also, the characteristic of impaired recycling makes itdifficult to explain the reported accumulation of this receptor in the nuclei of the cellsexpressing it. The phenomenon was reportedin aggressive tumors [21] It is note worthy that nuclear accumulation of various receptors has suggested being achieved through the endosomal pathway [14] Also, the new mechanism could explain the activation ofnuclear receptors such as AR in the absence of the ligand, which represents the maincause of theresistance to hormonal therapy, as well as the generation of the castrationresistant prostate cancer (CRPC). The accompanied figure demonstrates the role ofextracellular vesicles in transporting AR to the nucleus ofother cells and the activation ofthe AR responsive gene i.e. prostate specific Antigen (PSA) (Figure 1).
Figure 1: Cancer cells shed EV containing AR receptor to be transported directlyto the nucleus ofAR-nullcells.The transported receptor binds DNA of the acceptor cellsand stimulated transcriptionof the ARresponsive gene of PSA, and stimulate the releaseof PSA.
The roleof extracellular vesicles in intercellular exchange of membrane receptor [15,16] captured the attention of thescientific community [22] and is considered alandmark in cancer cell biology. Transporting exogenousreceptors to the nucleus ofother cells to be in direct contact with the DNA of the host cell, and the induction of thereceptor’s responsive genes represent a new level of understanding in cancer cell biology andestablishes a new vision for the complexity of the cancer microenvironment. It showsthat cancer cells canhijack the transcription machinery of other cells, which might bestromal cells. By hijacking the transcriptionmachinery of other cells, cancer cells mighthave a nourishing microenvironment. Also, by this mechanism,cancer cells may acquirecytoprotective characteristics to overcome the effect of various anticancer therapies, andmetastatic characteristics as well [23] It is noteworthy that cancer cells may developmechanismssimilar to the ones performed by the viruses to utilize the host genome toreplicate and produce their molecular components. Interestingly, there are studiessuggesting that extracellular vesicles are of viral origin [24] Revealing various mechanisms adopted by cancer cells will bring us closer to understanding the challengingnature of this devastating disease. This will lead to new means to disarm cancer cellsfrom theseremarkable capabilities and mechanisms and increase their responsiveness totherapies.


