Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Johannes Matiasek* and Leonhard Gmelch
Received: May 25, 2018; Published: May 31, 2018
*Corresponding author: Johannes Matiasek, Department of Plastic and Reconstructive Surgery, St. Josef Hospital, Vienna, Austria
DOI: 10.26717/BJSTR.2018.05.001150
The global threat posed by antibiotic resistance is well recognized, but despite, guidelines, antibiotics continue to be over-prescribed and frequently misused. One area where antibiotics may be used with considerable less frequency is in the management of infected wounds, particularly where the infection is localized and not systemic. This paper presents the case for considering the use of octenidine-based antiseptics for both wound care and whole-body decontamination, in order to prevent wound infections as well as to treat already locally infected wounds. A case report is presented which examines the management of a superinfected wound without the use of systemic or local antibiotics. Complete wound closure was achieved using a combination of octenidine based products.
Keywords: Superinfection, Antibiotic resistance, Antibiotic-free therapy, Octenidine
According to the World Health Organization (WHO) [1], antibiotic resistance is one of the biggest threats to global health today. A growing number of infections are becoming harder to treat as the antibiotics used to combat them become less effective. Antibiotic resistance leads to longer hospital stays, higher medical costs and increased mortality, therefore a significant change in the prescribing and use of antibiotics is required [2] (as well as the use of antimicrobials in livestock). By adhering to current antibiotic prescribing guidelines and reporting antibiotic-resistant infections to surveillance teams, healthcare professionals can contribute towards the control of resistance. Recently published guidelines on professional wound management, recommend that topical treatments based on effective antiseptic molecules like iodine, polyhexanide or octenidine should be used first line to treat infected wounds [3].
These antiseptic molecules have an unspecific mode of action making antimicrobial resistance unlikely and to date no resistance has been reported. A case report is presented in this article about the management of a superinfected wound without the use of systemic or local antibiotics. This management method should be considered for infected wounds where no clinical signs of systemic infection are diagnosed. It may also be considered as part of a wound infection prevention protocol.
Following excision of a squamous cell carcinoma on the left lower leg, a 73-year-old male patient received a split-thickness skin transplantation to cover the wound. Apart from type 2 diabetes, controlled with oral medication, the patient had no other comorbidities. The tie-over dressing was removed on day 6 while the graft was still intact, but the wound showed early signs of infection. Initial topical treatment was with medical honey in combination with a gauze dressing. This was changed on alternate days in the hospital outpatients’ department. Due to necrotic regions within the graft, the wound did not heal and the patient presented in our clinic with a superinfected wound on day 16 post surgery. The wound was moderately painful (pain score 5/10), malodorous and showed low exudate level. The complete wound measured 12 x 8.5 cm. Swabs were taken and the following pathogens were detected: E.coli (ESBL, Extended-Spectrum Beta-Lactamase), S.aureus (MRSA, Methicillin-resistant S.aureus), P.vulgaris, P.aeruginosa and E.faecalis.
As the patient did not present with any signs of systemic infection, it was decided to commence local therapy without concomitant systemic antibiotics. This was because the wound was already contaminated with antibiotic-resistant Gram-positive as well as Gram-negative bacteria. Gauze dressings were soaked with octenisept® (active ingredient octenidine; Schuelke & Mayr GmbH, Germany), a liquid medicinal product for wound antisepsis, and applied for between 3-4 minutes on the graft to loosen encrustations as well as to reduce the bacterial bioburden in the wound. After debridement of the wound bed and additional cleaning with octenisept®, octenilin® (an octenidine-based hydrogel, Schuelke & Mayr GmbH, Germany) was applied to moisten the granulation tissue. The wound was then covered with a hydrofiber dressing (Durafiber, Smith & Nephew GmbH, Germany). Because of the high grade of infection, the dressing was changed daily for 5 consecutive days and thereafter every 2 days.
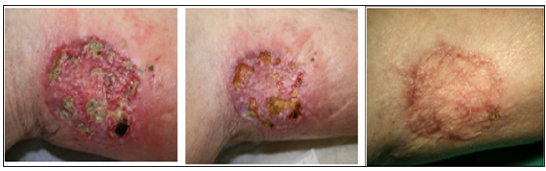
In addition to wound care the patient undertook total body decontamination for 5 days using octenisan® body wash and octenisan® md nasal gel (both octenidine containing products, Schuelke & Mayr GmbH, Germany) to reduce the risk of recontamination and to help to increase the success of topical wound therapy. After 5 days only P. aeruginosa was still present in the wound. The patient reported a cooling effect on the wound area, the dressing was less sticky when it was changed and there was an improvement in the pain score (2/10) only 4 days after initiation of octenilin® therapy. The wound as well as the surrounding skin appeared less inflamed and there was no maceration detectable during the whole treatment period. The wound bed was fully debrided and covered with granulation tissue on day 33. At 2 weeks of octenidine wound treatment a swab showed that Pseudomonas spp. was no longer detectable. Wound treatment continued until complete wound closure occurred on day 42. The total octenilin® treatment duration was 24 days (Figure 1).
Figure 1A: infected wound (day18), B: treatment with octenidine-based products for five consecutive days (day 22), C: complete wound closure (d42).
Antibiotic consumption is known as a primary driver of antibiotic resistance [4] and at the same time a global increase of its use during the last decade was recently reported, mainly by lowand middle-income countries [5]. Therefore, antibiotic resistance is recognized as a major global threat to health. It has been attributed to both the misuse and the overuse of antibiotics, together with a lack of new drugs [6]. In the management of wounds, the use of topical or systemic antibiotics may not always be necessary. Some wound infections may be prevented by the use of antiseptic substances like silver, hypochlorous acid, iodine, polyhexanide or octenidine. If a wound only shows clinical signs of localized infection, topical treatments based on iodine, polyhexanide or octenidine may be considered first line, rather than using an antibiotic.
In the department of Plastic and Reconstructive Surgery at St. Josef Hospital, Vienna, octenidine-based products are the first line treatment for both preventing wound infection and for the treatment of locally infected wounds. Octenidine has a broad spectrum of activity [7], including emerging multi-drug-resistant Gram-negative pathogens [8]. In a biocompatibility index test to assess the suitability of an antiseptic agent in clinical practice, both the microbicidal activity and the cytotoxic effects were examined. In this test octenidine showed an outstanding biocompatibility index compared to other antiseptics [9]. Octenidine is safe and well tolerated, even by the most vulnerable patients including neonates [10]. Furthermore, octenidine, unlike iodine, is colorless, which is an additional advantage when evaluating the healing of a wound when the dressing is changed. Additionally, all octenidine-based products can be utilised in combination which each other without any adverse interaction. Octenidine is widely used, particularly in Europe and Asia for wound care in the healthcare environment, as well as for patient decolonization [11,12].
In the case study presented, a diverse spectrum of bacteria was identified, with some already showing resistance to standard antibiotic drugs. The patient did not show signs of systemic infection, if he had, there would have been a risk of serious complications due to his underlying diabetes. Therefore, total body decontamination was undertaken as well as wound disinfection. Although patient decolonization is not standard practice in routine wound care, this additional measure was carried out concomitantly to help increase the success of the wound therapy and also to avoid recontamination. A combination of mupirocin and chlorhexidine is commonly used to decontaminate the nasal passages and the body. Since a massive number of Gram-negative species were found, it was decided to use octenidine rather than chlorhexidine for patient decolonization. In addition, better skin tolerability is reported for this molecule compared to chlorhexidine [13]. Resistance to mupirocin [14] is increasing worldwide, while octenidine is effective against such mutants [15].
In conclusion, by using octenidine for wound management and decolonization, a superinfected wound was effectively treated without the use of any antibiotics. Therefore, this approach could be tried in other healthcare settings where a wound only shows signs of localized infection.


