Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Percília CL Alves*1, Adriana L Oda1, Ana L C Vecina1, Juliana W C Neves1, Rosana M Borges1, Helena N M Sierra1, Marco Orsini2, Maria SG Rocha3 and Acary S B Oliveira1
Received: April 28, 2018; Published: May 07, 2018
*Corresponding author: Percília CL Alves, Rua Capitão Pacheco Chaves, 348 ap.1101. Vila Prudente. São Paulo/SP, Brazil
DOI: 10.26717/BJSTR.2018.04.001038
Objective: Analyze the interaction between dysphagia and the nutritional implication on Neuron Motor Disease patients.
Method: Through observational, transversal, prospective and analytical research, 59 patients, 42 (71,18%) were patients with Amyotrophic Lateral Sclerosis (47,62% males and 52,38% females) and 17 (28,82%) were patients with Progressive Bulbar Paralysis (7 (41,17%) males and 17 (58,83%) females). Patients underwent dysphagia and nutritional assessment and other scales like: Functional Oral Intake Scale, Functional Rating Scale (ALSFRS-R and EGELA) and respiratory assessment (cough peak flow).
Results: 100% of the patients with bulbar involvement showed changes in oral and pharyngeal phases, while ALS patients showed changes in oral phase (66,67%) and pharyngeal phase (73,80%). The nutritional assessment showed malnutrition in 35,71% of the patients with ALS and 23,52% of the patients with PBP. Patients who used other alternative type of feeding: 28% (ALS) and 41,17% (PBP).
Conclusion: Dysphagia was present in all of the patients with bulbar involvement, relating largely with malnutrition. Body Mass Index and Score DEP showed correlation to function rating scale ALSFRS-R. There was correlation between Body Mass Index and the Functional Oral Intake Scale and the cough peak flow.
Keywords: Amyotrophic Lateral Sclerosis, Dysphagia, Nutrition assessment, Speech, Language and Hearing Sciences
Abbreviations: ALS: Amyotrophic Lateral Sclerosis, UMN: Upper Motor Neuron, LMN: Lower Motor Neuron, BMI: Weight Loss or Body Mass Index, PBP: Progressive Bulbar Palsy, IFC: Informed Consent Form, FOIS Scale: Functional Oral Intake Scale, PEMS: Protein Energetic Malnutrition Scale
Amyotrophic Lateral Sclerosis (ALS) is a neuromuscular disease characterized by the progressive degeneration of upper motor neuron (UMN) and lower motor neuron (LMN). When this degeneration gets to the brainstem motor nuclei, patients evolve to dysphagia, dysphonia and dysarthria [1]. Worldwide incidence ranges from 1,5 to 2,7:100,000 people/year. Masculine gender is more affected in a proportion of 2:1 [1,2]. The average age varies between 55 and 65 years old. The bulbar ALS onset is more common in elderly women [1]. After the onset, patients may develop dysphagia, malnutrition and dehydration. The speechlanguage pathologist intervention includes specific orientation and adjustment throughout the evolution of the underlying disease and the level of dysphagia; food consistency is modified and consequently, a reduction in the amount of food intake of high protein-energy content might be necessary, especially on patients with bulbar dysfunction [3]. Therefore, the total amount of daily food intake, may be qualitative and quantitative insufficient to meet these patients’ needs, which makes nutritional supplementation necessary [3-7]. Weight loss or body mass index (BMI) reduction is relevant indicators of malnutrition and negative predictors of survival on ALS/NMD [8-9]. It is likely that anthropometric measurements may reflect the nutritional condition, also the loss of motor neuron [3]. The reduction of nutritional intake is multifactorial, determined by inappetence, dysphagia, weakness, dyspnea and depression [5,7,10-13]. The goal is to analyze the interface between dysphagia and the nutritional implication on NMD/ALS patients.
It is a field research, observational, transversal, prospective and analytical. There were 59 patients with Neuromuscular Disease evaluated, 42 (71.18%) had Amyotrophic Lateral Sclerosis (ALS) and 17 had Progressive Bulbar Palsy (PBP).The selection of patients for this research was based on the criteria described below.
Inclusion Criteria: Patients included in this study were followed up by a multidisciplinary UNIFESP Sector of Neuromuscular Disease Investigation. Patients from both genders, older than 18 years of age, with clinical manifestations and defined neurophysiological criteria of NMD/ALS according to criteria of El Escorial (1998) with or without complaints referent to swallowing problems, with or without bulbar musculature dysfunction
Exclusion Criteria: Patients who weren’t treated according to pre-defined protocols and patients who showed different neurological diagnose were excluded. After agreeing to take part of this study, patients read and signed the Informed Consent Form (IFC). Patients underwent the following assessment procedures:
a) Speech language pathology evaluation,
b) FOIS Scale (Functional Oral Intake Scale)
c) Cough Peak Flow,
d) Nutritional Assessment,
e) Functional Rating Scale ALSFRS-R and EGELA.
The speech language pathology evaluation consisted of directed anamnesis taking into consideration complaints presented. Functional anatomic aspects of the cervical region, face and phonoarticulatory organs were evaluated by the observation of postural, symmetry, tonicity, mobility and strength anatomy, in resting position and spontaneous and intended movement. A muscular clinical assessment was conducted: orbicular muscle of the lips, tongue and buccinators, soft palate and masticatory mucosa.In addition, deglutition and masticatory jaw movement functions were evaluated.
Mastication: Mastication was observed regarding lip posture; strength, amplitude, speed and direction of the movement; cut and lateralization of bolus; associated movements and masticatory efficiency. It was used a stale bread roll as an evaluation tool, because of its adequate consistency to the observation of components mentioned above.
Deglutition: Events of oral phase and pharyngeal phase of deglutition were observed, while offering different consistencies: thin liquid, nectar, honey, pasty and solid and in different volumes: 3ml, 5ml and 10ml. Patients were categorized according to the levels of Functional Oral Intake Scale (FOIS) [14], considering dietary characteristics, based on the properties and texture of food.
Nutritional Evaluation For the patient’s nutritional evaluation, anthropometric measurements were used: body weight (kg), height (cm), arm circumference (cm), muscle mass, tricipital skinfold (mm2), arm muscle area (cm2). The nutritional condition was categorized according to the Body Mass Index (BMI – ratio of body mass and height as an indicator of body mass index BMI = body mass/height2) and Protein Energetic Malnutrition Scale (PEMS).Functional Scale
Functional rating scales were used: ALSFRS-R and EGELA the Amyotrophic Lateral Sclerosis Functional Rating Scale – Revised) is a scale based in a questionnaire for daily activities. This scale has 12 groups of topics in three different aspects which comprises appendicular function (motor tasks), bulbar and respiratory function. The Scale of Severity for ALS (ALSSS) is a scale based on a questionnaire for activities related to four main aspects: lower limbs, upper limbs, speak and deglutition. For scales, the lower the score, the higher the severity of the disease. Statistical Analysis. The scores of the evaluation methods used were compared to different groups according to the Mann-Whitney test. The correlation between the continuous variables were evaluated with the Spearman test. Findings were considered statistically significant, those with rate p or with error probability type I below 5%.The research project was evaluated and approved by the Ethics Committee in Research by the Universidade Federal de São Paulo, registered under the number 1450/11
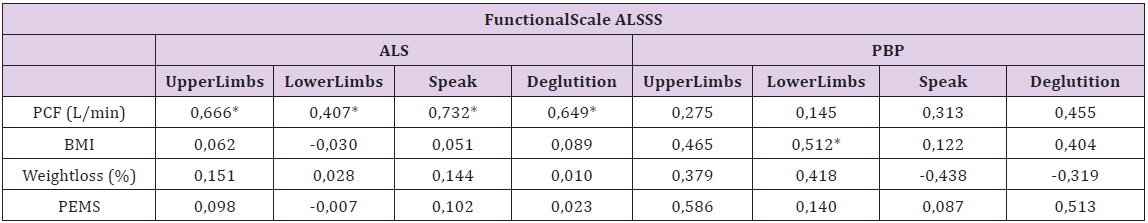
The time of diagnosis was 16.5 (2-114) months for the ALS group and 9.0 (0-29) months for PBP.There was no substantial statistical different on nutritional indicators between the two groups (Table 1) Oral diet only and nutritional diagnose of Eutrophia were present more frequently on ALS patients (Figures 1 & 2) shows the distribution of values of Peak Cough Flow (PCF) and FOIS according to each diagnosis group, not taking into account the significant statistic difference of PCF between ALS and PBP groups. Patients with ALS showed better scores on FOIS scale. Oral phase of the deglutition was affected in the majority of the patients, it was observed in approximately 66% of the ALS patients and all of the PBP patients.In general, all of the events of oral phase were more frequently affected on PBP patients (Table 2). The pharyngeal fase of the deglutition was affected in the majority of the patients, aprox. 74% of the ALS patients and all of the PBP patients. In general, all of the events in the pharyngeal phase were more frequently affected in the group of PBP patients (Tables 3 & 4) shows values of the coefficient of bivariate analisys between ALSFRS-R, ALSSS and the PCF indicator according to the score obtained on the FOIS scale. All of the analised aspects showed correlation directly proportional and significant for the FOIS scale between patients of the ALS group, ranging between 0.39 and 0.91. Whereas patients with PBP didn’t show significant correlation on these aspects and the score on FOIS scale, except on the Deglutition aspect of the ALSSS scale with coefficient close to 0.89.
Figure 1: Oral diet only and nutritional diagnose of Eutrophia were present more frequently on ALS patients.
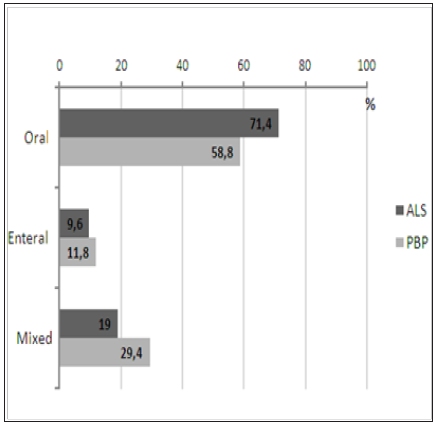
Table 2:Frequency of events of the clinical evaluation of theoral phase according to diagnostic group.

Table 3:Frequency of events of the clinical evaluation of the pharyngeal phase according to diagnostic group.
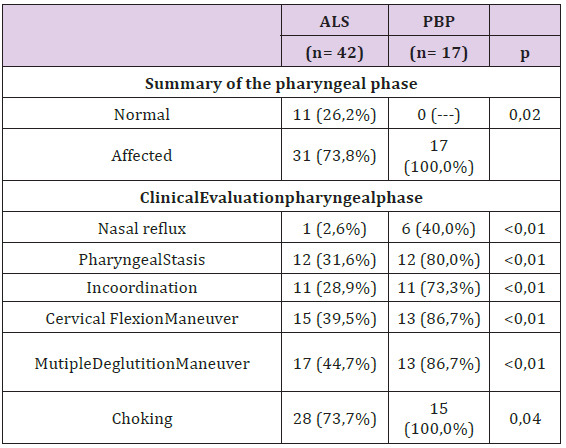
Table 4:Correlation Matrix (Spearman) between, ALSFRS-R, EGELA scales and the Peak cough flow according to diagnostic group.

There were significant correlations between the PCF and the ALSFRS-R scale aspects in ALS patients, with coefficients ranging from 0.41 to 0.61. Among patients in the PBP group, only the total scores on the ALSFRS-R scale correlated positively with the PCF. It is interesting to note that no significant correlations were found between nutritional indicators and the ALSFRS-R scale among patients in the ALS group, despite the significant findings between the BMI and the PEMS score with the total ALSFRS-R score in the patients in the PBP group. BMI also correlated significantly with the ‘appendicular’ and ‘respiratory’ domains of the ALSFRS-R scale in the PBP group (Tables 5 & 6). There were significant correlations between the PCF indicator and the aspects ALSSS scale, with coefficients ranging between 0.40 and 0.73 in ALS patients. On the anthropometric indicators, only the BMI and the ‘lower limbs’ aspect showed a significant correlation (r = 0.512, p <0.05).
Table 5:Correlation matrix (Spearman) between the ALSFRS-R scale and the nutritional anthropometric and phonoaudiological indicators with Peak Cough Flow, according to the diagnostic groups.

Table 6:Correlation matrix (Spearman) between the ALSFRS-R scale and the nutritional anthropometric and phonoaudiological indicators with Peak Cough Flow, according to the diagnostic groups.
ALS is a rare disease, with a global incidence around 1.5 to 2.7: 100,000 people/year [2]. The population studied presented a predominance of the appendicular form in relation to the bulbar form, which agrees with the epidemiological characteristics of the ALS / NMD [1,2]. The diagnosis of patients with ALS was later than the patients with PBP. The presence of the bulbar symptom seems to be a factor of better targeting of the clinical reasoning for the conclusion of the diagnosis of ALS / MND. This reinforces the importance of the professional in observing the functions of voice, speech, breathing and swallowing; as well as the differentiation of the onset of symptoms, between progressive and sudden [15]. Regarding nutritional indicators, there was no statistical difference between the appendicular and bulbar groups. Arm anthropometry is directly related to respiratory impairment and functional scale (ALSFRS-R). Tricipital skinfold measurement should be more valued for nutritional assessment, showing a predictor of motor and nutritional impairment [4].
In the comparative analysis of the nutritional status evaluation between electrical bioimpedance and anthropometry, no significant differences were observed between the methods, in which the anthropometric measurements alone would be sufficient for analysis of the body composition. Anthropometry may reflect both the nutritional status and the loss of motor neurons; both situations, however, are likely to be influenced and may contribute to disease progression [12]. As to the nutritional diagnosis, there was a higher frequency of malnutrition in bulbar patients. Patients with PBP presented a greater impairment of nutritional status in a faster way in comparison to those with ALS, justifying the early nutritional evaluation in these patients. On the other hand, patients with ALS present impaired early nutritional status prior to the onset of dysphagia. Nutritional impairment is more severe comparing clinical manifestations in the form of PBP than in patients with the classic form of ALS [4].
NMD patients progress with weight loss because they present symptoms of dysphagia, necessities of dietary adaptations, such as reduced food intake, prolonged meals, dehydration, weakness, increased energy expenditure, even to maintain respiratory function and even, depression.Overweight and obesity were also observed in a smaller portion of the studied population. This may be due to previous eating habits and sedentary lifestyle. It is worth noting that the nutritional classification adopted, given by BMI, does not evaluate the composition of fat mass and lean mass of the patient, so that the eutrophic or overweight patient may present some degree of malnutrition. The indication of the nutritional supplement is aimed at the maintenance of lean body mass and the spontaneous increase of food intake [7]. In addition, the dysphagic patient tends to ingest more carbohydrates, due to difficulty in oral phase, lack of masticatory efficiency and / or difficulty in the pharyngeal phase. During the evolution of NMD, the progressive dysfunction of the oropharyngolaryngeal musculature and respiratory muscles, caused by the degeneration of motor neurons of the corticobulbar tract, affects the patient resulting in a complaint of dysarthria, dyspnea, dysphonia and dysphagia [15]. Patients with ALS are able to maintain exclusive oral passage longer than patients with PBP, but even patients with enteral feeding PBP are less eutrophic, probably because of energy consumption to maintain neurovegetative functions such as breathing, chewing, sucking and deglutition.
Modification of food consistency, necessary for dysphagia that worsens during the course of the disease, contributes to the exclusion of high-energy foods. In the analysis of food intake, it was verified that only one of the 16 patients studied presented an adequate food intake against caloric intake recommendations [5]. Patients who require changes in food consistency should receive nutritional and clinical follow-up, since the total daily food supply may be qualitative and quantitatively insufficient to meet the needs of such patients, making supplementation necessary [3]. In the application of the FOIS Scale, on food consistency, the index was higher for patients with ALS and lower for patients with PBP; indicating that the severity of dysphagia is mandatory in the choice of food consistency. The dynamics of the musculature involved in swallowing in ALS occurs with greater amplitude of movement, strength compared to PBP, causing impact on food consistency, being important to emphasize that speech and swallowing are the same structures, but with different refinement depending on the level of dysphagia, determining differences in volume and food consistency.
In the present study, the majority of patients with PBP received a single consistency, depending on the degree of impairment of the musculature involved in swallowing. In patients with ALS, the majority received multiple consistencies, being compatible with peak cough flow more effective for the management of the functions, but both groups present some degree of dysphagia. The FOIS Scale is considered an evolution indicator, regarding the functionality of swallowing [16,17]. A national study demonstrated a change in the levels of FOIS during the follow-up of a group of patients with ALS compared to a group with Parkinson’s Disease. The difference between the two groups of patients was remarkable. In patients with ALS, the functionality of swallowing worsened, being aggravated by an average of 2.5 FOIS levels during the follow-up period [18]. In another study with neurological patients, the speech-language intervention contributed to the clinical and pulmonary improvement of the patients, with reduction of the episodes of laryngotracheal penetration and / or aspiration, by verifying the possibility of oral intake, using the said scale [16]. Diets modified with fortified texture for dysphagic patients can accelerate weight loss due to their low protein and energy content.
When these factors are present the insidious onset of malnutrition is inevitable.It is important that the professional is careful to classify oropharyngeal dysphagia for the different consistencies tested, since one patient may be dysphagic for one consistency and not for another. The penetration or aspiration episodes are not always the indicators of dysphagia severity, as most of the classification proposals suggest, since the occurrence of stasis, posterior and anterior loss may be as indicative of the severity of aspiration dysphagia and future Respiratory compromise, such as choking [15]. As the disease progresses the orofacial muscles, pharynx, larynx, respiratory muscles are also affected resting or in movement and in the functions of suction, swallowing, chewing, speech, voice, breathing and in the association of both functions, caused by neuronal degeneration Motors and the corticobulbar tract. In a study with videoendoscopy examination of swallowing, the patients with ALS/PBP presented some altered swallowing phase. The presence and the moment of laryngeal penetration or tracheal aspiration with food in the liquid consistency in more than 90% of the patients during the pharyngeal phase of deglutition [19].
Dysphagia raises the index of a cascade of comorbidities, leading to risk of bronchoaspiration, malnutrition, dehydration and, consequently, death.Speech-language management should be initiated from the earliest symptoms to the most advanced stage of the disease in order to minimize/avoid dysphagiarelated complications related to nutrition and respiration. Speech language pathology includes changes in food consistency, such as the use of thickeners, as well as compensatory procedures acquired through myofunctional exercises and learning techniques that stimulate oral proprioception, postural changes and swallowing maneuvers [20-22]. Oropharyngolaryngeal weakness affects the survival of individuals with ALS in two ways. First, through a continued risk of aspiration pneumonia and sepsis. Second, through inadequate intake of energy and protein [5]. Weakness of tongue musculature has been described as a predictor of survival in patients with ALS [15,23]. Patients with PBP should be evaluated more frequently compared to patients with ALS, due to the need for phonoaudiological adaptations of the swallowing and communication dynamics, in view of the greater impairment of the musculature of the stomatognathic system [24].
There was no difference in the performance of both groups, regarding peak cough flow; which corresponds to the resemblance in the respiratory part, evidenced in the ALSFRS-R functional scale. However, the average is below 200 liters per minute, since it is below that required for good airway protection. Peak cough flow is directly correlated with the ability to eliminate secretions from the respiratory tract, and values below 160L / min are associated with inadequate tracheobronchial tree cleaning. However, higher values, however close to 160L/min, do not necessarily guarantee adequate airway protection, since muscle strength tends to worsen during infectious episodes [25]. For this reason, a peak cough flux value of 270L / min has been used to detect patients who would benefit from assisted cough techniques [26]. Weakness of the expiratory musculature combined with inadequate lung insufflations prevents efficacy of coughing and clearance of airways, alternating airway resistance and increasing the risk of developing atelectasis and pneumonia [27].
It is important to correlate neurogenic oropharyngeal dysphagia with respiratory pictures, being an effective cough reflex for the help and management of secretions and, consequently, essential for minimizing and even avoiding bronchoaspiration. To know the factors or the combination of factors that could increase the risk of a possible tracheal aspiration and how to avoid it allows to establish dietary changes, use of swallowing maneuvers, indication of alternative feeding routes and oral suspension when necessary [28,29]. In patients with ALS there is an increase in basal energy expenditure, which contributes to the loss of body weight, which in turn is a determining factor for survival. The 5% decrease in usual weight at the time of diagnosis increased the risk of death in the studied population by 30% [9]. It is also important to consider the question of hydration, with nutritional aspects, since hydration is a survival factor. In a previous study, the supplemented patients presented weight gain, increased body mass index, increased arm muscle area and circumference, among other biochemical alterations [13].
The fact that there is a positive correlation between lower limb severity scale and BMI indicates the need to perform an early nutritional intervention, from early stages of the disease, where there is only evidence of motor impairment, reducing the frequency of changes in body composition and Consequences of the evolution of ALS / NMD [4]. Because it is a disease that consumes a lot of energy, maintaining or trying to avoid weight reduction provides a protective factor [9]. Currently, the indication of PEG is being made as early as possible.It was observed that there are important differences in several aspects studied between the ALS and PBP groups. For this reason, it is worth mentioning that during the treatment of the patient with ALS and PBP, the professional should be aware of the particularities of each disease. In the same way, the speech-language intervention in the swallowing dynamics of patients with motor neuron disease should have a differentiated look, providing therapeutic gain for the patient, family / caregivers and team.The treatment of ALS / NMD is palliative, requiring the performance of a multidisciplinary team, which will support both the patient and the caregiver. Multidisciplinary care models have developed as a predictor of survival reducing the risk of death by 45% in five years when compared to patients treated at general neurology clinics [30].
Dysphagia was present in all patients diagnosed with PBP, related to a higher frequency of malnutrition in this population.In the presence of bulbar symptoms, there was a correlation between body mass indexes and the DEMS with the ALSFRS-R functionality; as well as a correlation between body mass index, consistency scale (FOIS) and peak cough flow.
We thank all the patients, for the encouragement in researching rare diseases.


