Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yoshio Ueda, Yu Sueyoshi, Takuya Nakagoshi, Noritaka Isogai* and Hirohisa Kusuhara
Received: April 09, 2018; Published: April 18, 2018
*Corresponding author: Department of Plastic and Reconstructive Surgery, Kindai University Faculty of Medicine, Osaka-sayama, Japan
DOI: 10.26717/BJSTR.2018.03.000975
Keywords: Biodegradable polymer; Nerve Conduit; Nerve Regeneration; Diameter
In the treatment of peripheral neurological deficits, autologous nerve grafting is the first choice. The sural nerve, ante brachial cutaneous nerve, terminal branch of posterior interosseous nerve, and other choices are commonly used, however, loss of function and potential morbidity from creation of these donor sites are undesirable effects of their use. In recent years, synthetic nerve conduits and allogeneic nerve grafting materials have been introduced into clinical practice as alternatives to autologous nerve grafting [1-3]. The first attempt at nerve conduit was application for nerve regeneration using silicone tubes [4] Subsequent studies explored biodegradable materials with open internal structure as the conduit. More recently, filling the conduit with a regeneration- conducive material such as collagen demonstrated improved nerve regeneration, however, the outcome remained inferior to autologous nerve grafting and allergenic nerve transplantation [5-7]. To promote nerve regeneration, biodegradable nerve conduits need to be carefully sutured to the donor nerves at the anastomotic site as the poor adaptation often allows fibroblasts invasion into the lumen of the conduit which hampers nerve regeneration. Unfortunately, no study has elucidated the influence of diameter discrepancy between the biodegradable nerve conduit and the donor nerve for better nerve regeneration. In this study, we introduced biodegradable nerve conduits of different diameter to examine how the diameter discrepancy influences nerve regeneration.
Figure 1: Scanning electron microscopic findings of biodegradable nerve conduit (A) and macroscopic finding after implanta-tion to the sciatic nerve gap (B).
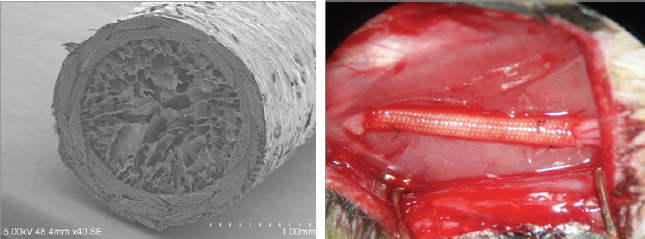
Observation of the surface of biodegradable nerve conduit was conducted using scanning electron microscopy. The samples were dried and gold-coated with evaluation using a scanning electron microscope (S-900, Hitachi, Ltd., Japan) (Figure 1A).
All the animal experiments were conducted following the regulations of the Animal Experiment Committee of Kindai University. Fourteen-week-old SD rats (mean body weight 280~324 g Harlan Sprague Dawley, Indianapolis, IN, USA) were used as experimental animals (n =18). Housing was maintained in a clean rack with uniform temperature (22°C) and humidity (50%) and 12-hour light-dark cycle. Radiation-sterilized (3 mG) solid food and water was provided ad libitum. After general anaesthesia with urethane and a-chloralose, a 10-mm gap was created by sharp transection of the sciatic nerve on one leg per rat. To fill the gap, 12 mm biodegradable nerve conduit was then implanted. The ends of the nerve were drawn into the open ends of the biodegradable nerve conduit by 1 mm on each side using a horizontal mattress stitch of 10-0 nylon suture to secure each end in place (Figure 1B). Biodegradable nerve conduits of three different diameter (1.5, 2.0, 2.5 mm, respectively) were implanted (n=6 in each group). Evaluation of nerve regeneration was done 12 weeks after the surgery (Figure 2).
At 12 weeks after implantation, the biodegradable nerve conduit was harvested and the tissues were immersion-fixed in 10% neutral buffered formalin solution for 3 days. Paraffin blocks were prepared and 5-|im sections were made in the longitudinal direction with a microtome (LEICA SM2000R). Toluidine blue was used for staining area of nerve regeneration.
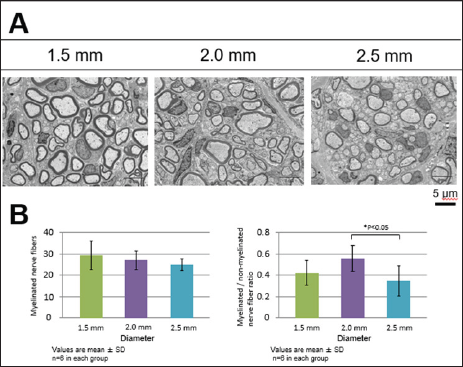
Biodegradable nerve conduits collected at 12 weeks after implantation were immersed and fixed in 4% glutaraldehyde for 3 days. The tissues were sectioned (1.5|im thick) transversely with an ultra microtome (LEICA EM UC7) and then post-fixed with 4% osmium. The slices were dehydrated by alcohol series, stained with uranium and embedded in peon. They were sliced to ultrathin sections of 700 A in thicknesses, stained with urinal and lead acetate and observed using a transmission electron microscope (H-7700, Hitachi, Ltd., Japan). The sites observed was the middle region of biodegradable nerve conduit at the maximum scale possible to identify axons, 50 |im x 40 |im (= 2,000 |im2). Three visual fields near the center of each cross-section in each group were photographed, and the mean value per unit area of 2,000 |im2 was calculated for area of militated nerve fibbers and militated/non-militated nerve fiber ratio. Image J software was used for analysis.
All experimental results were indicated as mean ± standard deviation (SD). Statistical analyses used one-way analysis of 2/5 variance (one-way ANOVA) and Holm post-test for comparison. Software Inc., San Diego, CA, USA), and P<0.05 was used as the Data analysis was conducted using GraphPad Prism (GraphPad criterion for significance.
Every conduit maintained a clear profile within the tissue bed with little evidence of integration with the surrounding tissue. The nerve conduits became thinner in 1.5 mm group at 12 weeks after implantation. In contrast, 2.0 mm group showed clear thickening (Figure 3).
Figure 4: Histological evaluation of the biodegradable nerve conduit.
(A) The biodegradable nerve conduits implanted to the sciatic nerve gap were collected at 12 weeks in vivo. Middle region of the implanted conduit was toluidine blue-stained. (B) Area of nerve regeneration was measured.

The area of nerve regeneration in 2.0 mm group showed significant increase when compared with those of 1.5 mm and 2.5 mm group (Figure 4).
The density of militated nerve fibbers (number of fibbers per 2000 um2 area) in all three diameter groups was not found to differ, whereas militated/non militated nerve fibber ratio was statistically lower in the 2.5 mm group in comparison to 2.0 mm group at the respective level (p < 0.05) (Figure 5). It was lower in 1.5 mm group than that of 2.0 mm group, though this did not achieve statistical significance.
In the treatment of peripheral neurological deficit, the nerve conduit of hollow structure is indicated for the deficit within 2 cm, and for the neurological deficit longer than this, allogeneic nerve transplantation and autologous nerve transplantation are indicated [1-10]. To modify the biodegradable nerve conduit is clearly needed. The nerve conduit tested in the present experiment is of the biodegradable material obtained by forming PGA fibber to a luminal structure of which the thickness of luminal wall was adjusted to about 500 |im. Different from the biodegradable nerve conduit having conventional hollow structure, the inner lumen is filled with collagen. It is already reported that the collagen in the inner lumen becomes the scaffold in migration and growth of various cells contributing to nerve regeneration and the nerve regenerative ability is accelerated [11-17]. Moreover, the low- molecular-weight molecules such as albumin are able to penetrate through the biodegradable nerve conduit wall and, therefore, nerve regeneration is more excellent than the previous nerve conduit [16]. In this research, we prepared the nerve conduit prepared from biodegradable materials of different diameter to examine how the diameter discrepancy of the biodegradable nerve conduit influences nerve regeneration. As a result, it was found that, when the biodegradable nerve conduit of 2.0 mm diameter was implanted, the nerve regeneration and ratio of militated/non-militated nerve fibber were promoted at 12 weeks after implantation. This suggests that the optimum diameter of the biodegradable nerve conduit is about 1.5 times larger than the donor nerve to obtain better nerve regeneration.
Figure 5: Transmission electron microscopic findings of the biodegradable nerve conduit.
(A) Middle region of the implanted biodegradable nerve conduit was observed at 12 weeks after implatation using transmission electron microscope. (B) The area of myelinated nerve fiber (left) and myelinated/non-myelinated nerve fiber ratio (right) per 2,000 μm2 were evaluated quantitatively.