Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jagdish Singh*
Received: March 09, 2018; Published: April 10, 2018
*Corresponding author: Jagdish Singh, Principal Scientist & Head, Division of Basic Sciences ICAR-Indian Institute of Pulses Research, Kanpur-208 24, UP, India
DOI: 10.26717/BJSTR.2018.03.000940
Folate is a B vitamin that occurs naturally in foods such as green leafy vegetables, citrus fruit, and beans. Folic acid is man-made (synthetic) folate. It is found in supplements and added to fortified foods. Folate has many functions in the body, with vitamin B12 and vitamin C it helps the body to break down, use, and create new proteins, helps to form red blood cells and prevents anemia and also helps to produce DNA, the building block of the human body. Rich sources of folate include Spinach, Dark leafy greens, Asparagus, Turnip, Beets, Mustard greens, Brussels sprouts, Lima beans, Soybeans, Beef liver, Brewer's yeast, Root vegetables, Whole grains, Wheat germ, Kidney beans, White beans, Lima beans, Mung bean, Salmon, Orange juice, Avocado and Milk. Folic acid is of critical importance periconceptionally in protecting the foetus from neural tube and other congenital defects. Folate deficiency is a global problem affecting millions of people in both developed and developing countries. Inadequate intake of folic acid during pregnancy increases the risks of preterm delivery, low birth weight, foetal growth retardation, and developmental neural tube defects (NTDs). In addition, low folate intake and elevated homocysteine levels are associated with the occurrence of neurodegenerative disorders, cardiovascular diseases, and a range of cancers, while adequate intake of both folates and folic acid in diets decreases total homocysteine levels in plasma. Folic acid fortification and supplementation approaches have been adopted in many parts of the world, largely due to folate bioavailability issues and also safety concerns regarding excess folate exposure in vulnerable population groups (e.g., children). Thus, alternative approaches to supply folates through biofortification of staple food crops may provide a sustainable means to provide bioavailable folates to people in many parts of the world. Most staple food crops, are poor sources of dietary folates, however, legumes have traditionally been considered as a good dietary source of folates. This chapter describes the genetic variability of folate content in legumes and the potential for genetic biofortification in these crops.
Keywords: Biofortification; Chickpea; Field Pea; Folates; Lentils; Tetrahydrofolate
Abbreviations: NTD: Neural Tube Defects; THF : Tetra Hydro Folate; RDA: The Recommended Dietary Allowance; MA: Microbiological Assay; LC: Liquid Chromatography; LC-FD: LC with Fluorescence Detection; FTHF : Formyl Tetra Hydro Folate ; FA: Folic Acid; MTHF: Methy Tetra Hydro Folate
Figure 1: Chemical structure of folate and derivatives Courtesy: Comprehensive Reviews in Food Science and Food Safety.

Folate is the bioavailable, natural form of a water soluble vitamin B9 found in a variety of plant and animal foods. Folic acid, is the synthetic form of the vitamin; it's primarily found in supplements and fortified foods. The structural difference between folic acid and food folate accounts for differences in bioavailability, with folic acid being more readily absorbed. Folic acid contains one glutamic acid, whereas, folate contains 2-7 glutamic acid (Figure 1). The Tetrahydrofolate (THF) is involved in one carbon transfer reactions in many organisms including humans [1]. It acts as cofactor in many metabolic functions including the biosynthesis of nucleic acids, metabolism of amino acids, methylation of hormones, lipids, proteins, and DNA [2,3]. It also helps in maturation of red blood cells and prevents anaemia. In plants, folates are vital for biosynthesis of lignin, alkaloids, betaines and chlorophyll, and are indispensable in photorespiration [4]. Humans cannot synthesize folates and thus depend upon plant and animal sources [3,5] (Figure 1). Folate deficiency poses serious problems in both developed and developing nations and can cause serious health issues including neural tube defects (NTDs), impaired cognitive function, and cardiovascular diseases [6-8]. The Neural Tube Defects (NTDs) are birth defects of the brain and the spinal cord. They occur when the neural tube, which later becomes the brain and the spine, fails to close properly. This happens very early in pregnancy, between the 17th and the 28th day after conception. A defect may occur in the upper or lower portion of the neural tube.
Figure 2: Spina bifida a condition that results when the lower part of the neural tube fails to develop pr

If the tube fails to close properly on the upper portion of the neural tube, a brain defect called anencephaly or another called encephalocele occurs. If it fails to close properly along the lower portion of the neural tube, a spinal defect called spina bifida occurs (Figure 2). The spina bifida damage may lead to muscle weakness, paralysis, and loss of bowel and bladder control. Further, tt is also associated with numerous neurodegenerative disorders, including Alzheimer's disease [9], and various cancers [10]. The Recommended Dietary Allowance (RDA) of folates is 400 mg for adults and 600 mg for pregnant women [11]. Folate-rich diets are recommended to pregnant women as they play an important role in various metabolic processes including nucleotide biosynthesis during cell division [6]. Insufficient folate consumption increases the risks of preterm delivery, low birth weight, and foetal growth retardation [12]. Besides their role in megaloblastic anaemia prevention in pregnancy, folates are also essential for human reproductive health [13,14]. Reported that seminal plasma folate was correlated with blood plasma folate and hence important in male reproduction. Folates are synthesized de novo in bacteria, fungi and plants. Like other vertebrates, humans fully depend on their diet for folate supply. Being an important component of human diet, plants constitute the main source of folates for human population.
Unfortunately, most staple crops such as potato, rice, cassava and corn are relatively poor in folates; hence, in regions where these staples are the main (or sole) energy source, folate deficiency is highly prevalent [15]. Two approaches which are employed to fight folate deficiency include pharmacological supplementation in the form of folate pills and biofortification of staple crops. As the former approach is considered costly for the major part of the world population, biofortification, i.e., enriching the nutritional value of staple crops, is a balanced and economic way to improve the health status of low income consumers [15-17]. Bio-fortification is a feasible means of reaching malnourished populations in relatively remote rural areas, delivering naturally fortified foods to people in need. Although significant success has already been achieved in the enhancement of folate content in a number of plant species, such as rice, tomato and lettuce [18-20], but not much progress has been achieved in folate-rich crops like legumes. Therefore, strategies, challenges and genetic diversity studies for this phytochemical in such crops is highly essential. Understanding of these aspects is a prerequisite to the development of a successful biofortification strategy.
The wide range of folate derivatives, their high sensitivity to light, heat, and oxidation make folate analysis in foodstuffs quite difficult. There is a critical need for reliable methods to determine folate in foods to accurately estimate folate intakes in populations. However, current values for folates in foods in databanks are often underestimated due to the high instability of several folate forms, especially tetrahydrofolate. The two main methods utilized for folate estimation includes, microbiological assay (MA) and the second one by HPLC with different detection methods such as fluorimetric, UV-Vis, or mass spectrometric detection. A folate assay is generally divided into 3 steps: the extraction from the matrix, the deconjugation meaning cleavage of the polyglutamate chain by a Y-carboxy-peptidase (also called conjugase) and the purification prior to the quantification. For many years Microbiological assays were used for analyses of folic acid [21].
In these methods for sample preparation typically used conjugase for digestion, but recent methods now use a trienzyme (protease, a-amylase, and rat conjugase) treatment [22]. The trienzyme method showed significant increases in measured folates indicating that the use of conjugase alone underestimated folate levels [22]. Microbiological assays are still used to estimate total folates [23,24]; however, more recently, methods using liquid chromatography (LC) have been employed as LC allows for the detection of specific folate forms. LC with fluorescence detection (LC-FD) [25,26] and LC with mass spectrometry (MS) detection [27-31] are commonly used. Tandem MS methods (MS/MS) are extremely selective as both the parent ion and a specific fragment ion are required for detection, thereby greatly reducing chemical interference. In addition, the use of isotopically labelled internal standards enables very accurate quantification, as they will account for losses in sample preparation or degradation. Recently, [29] used the higher resolution and faster separation capabilities of ultraperformance LC (UPLC) to develop a UPLC-MS/MS method for analysis of folates in rice. This method has a short run time that allows for a more efficient analysis of the highly labile folates before they decompose in the auto sampler.
Table 1: Folate content in Cereal grains.

Source: USDA National Nutrient data base for Standard Reference.
Folates are found in high concentrations in wheat germ, yeasts, liver, which is the folate storage organ in mammals, some cereals, as well as in pulses and leafy vegetables. The major staple crops such as rice, maize, plantain and potato are low in folates (Table 1) (USDA-ARS, 2012). However, pulses and other legumes, as well as beef liver and green leafy vegetables such as spinach, asparagus, lettuce, and Brussels sprouts are rich in folates (Table 2) [32,33] reported that folate concentration in rice cultivars ranged from 11.0 to 51 μg/100 g with a mean of 26.0 μg/100 g. Among the four rice cultivars, the pigmented grain cultivar possessed twofold higher total folates than the other three non-pigmented grain cultivars. They have concluded that the average value of 100 g serving of rice grains could provide the amount of recommended daily allowance (% RDA) of dietary folates (6.5%) for adults, which ranged from 2.7-12.7%. Among the 5 individual forms of folates, 5-methyltetrahydrofolate was most abundant form in rice cultivars followed by 10-Formylfolic acid and folic acid [34].
Table 2: Folate content in green leafy vegetables.

Source: USDA National Nutrient data base for Standard Reference
Determined that Folate content in Cereal-grain food product using Trienzyme Extraction (employing rat plasma folate conjugase, a-amylase, and protease), followed by Combined Affinity and Reversed-Phase Liquid Chromatography. Total folate concentrations for unfortified white and wheat bread and rice was reported as 21.3 ± 0.69, 29.8 ± 1.94 and 10.8±0.57 mg/100 g of product, respectively. Further the effect of different cooking methods on folate retention in various foods was estimated [35] and it was reported that boiling for typical time periods resulted in only 49 % retention of folate in spinach (191.8 and 94.4 mg/100 g for raw and boiled spinach respectively), and only 44 % in broccoli (177.1 and 77.0 mg/100 g for raw and boiled broccoli respectively). Steaming of spinach or broccoli, in contrast, resulted in no significant decrease in folate content, even for the maximum steaming periods of 4.5 min (spinach) and 15.0 min (broccoli). Compared with raw values, boiling of whole potatoes (skin and flesh) for 60.0 min did not result in a significant change in folate content (125.1 and 102.8 mg/100 g for raw and boiled potato respectively), nor was there any effect on folate retention whether or not skin was retained during boiling. The folate losses during cooking and preparation are the result of a combination of thermal degradation and leaching of the vitamin into the cooking water (Table1).
Legumes play an important role in the traditional diet in several regions of the world [36], and are recognized as important food sources of folate (Tables 3 & 4) [37,38]. The USDA nutrient database shows that lentil (Lens culinaris L.) and common beans (Phaseolus vulgaris L.) are two legume crops that are rich in folates. Beans that are especially high in folic acid include pinto beans, lima beans, and kidney beans (Table 3). Common beans (Phaseolus vulgaris L.) contain high levels of folates. Even though common beans contain high levels of folate in general, folate contents may vary significantly among bean genotypes. Inheritance of folate content was studied [39] in the F1 hybrids of one-way diallel crosses among four dry bean genotypes selected from the two bean gene-pools. Taylor (cranberry bean), AC Elk (light red kidney bean) and Redhawk (dark red kidney bean) are from Andean gene pool and Othello (pinto bean) from Mesoamerican gene pool.
Table 3: Folate content in cooked bean seeds.
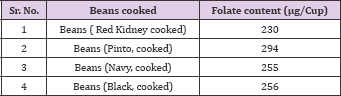
Source: USDA 2011 (www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/SR24/nutrlist/sr24a255)
Table 4: Folate content in mature seeds of legumes.
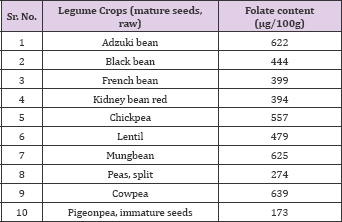
Source: USDA National Nutrient data base for Standard Reference.
Significant variation in folate content was observed among the parental genotypes, their F1 hybrids, and F2 individuals, ranging from147 to 345 μg/100g. The mean folate contents of Taylor and Othello were significantly (P< 0.05) lower than those for AC Elk and Redhawk. The estimate of GCA: SCA ratio ranged from 0.41 to 0.60 for different folate measurements, indicating that non-additive gene effects were more important than additive gene effects in the inheritance of folate content. The deviation of F1 from population mean and the mid-parent values, ranging from co-dominance to dominance, also indicated the possible involvement of non-additive gene effects. The total number of markers associated with at least one of the four measurements of folate content was four, distributed in three linkage groups. These QTL were on LG2, LG9 and LG11. Although most of these QTL do not have large effects individually, together these QTL could control a significant proportion of variation and may have larger effects in other genetic backgrounds [40] used the tri-enzyme extraction process to measure folate content in some selected Fijian foods. A total of 18 commonly consumed foods in Fijian diet were assayed, which included long beans (Vigna sesquipedalis L.).
In their study long beans contained 130 μg/100g of total folate [41] also measured the folate content of some Italian food items and reported that cooked beans contained 18.5 μg/100g of total folate, while cooked chickpeas contained 34.2 μg/100g of total folate (Table 3). Faba bean (Vicia faba L.) is important source of folate [38] estimated the folate content in faba bean and reported that the mean folate content in the green faba beans cultivars ranged from 110 to 130 ng 100 g-1 fresh weight or 535 to 620 ng 100 g-1 dry matter (DM), which was four-to six fold higher than in dried seeds. Industrial canning of dried seeds resulted in significant folate losses of approximately 20%, while industrial freezing had no effect. Germination of faba beans increased the folate content by > 40%. Although legume crops are very good source of folates, but it is not readily available due to complex binding with biomolecules (Neilson, 1994). According to [42], chickpea has higher content of folate compared with pea. Folate content in raw chickpea and pea were 149.7 and 101.5 ng 100 g-1, respectively, and 78.8 and 45.7 ng 100 g-1 in boiled chickpea and pea respectively, indicating that some folate may have leached in the water used for processing (Table 4). Although studies have been conducted to measure folate concentrations in various legume crops [26,27], however, information on the diversity in folate profiles of pulse crop cultivars grown in different locations is not available.
This diversity information can be used to determine the scope for future biofortification of folates in pulse crops through conventional or molecular breeding approaches [43]. Estimated the folate concentration in pulses by a microbiological method employing trienzyme extraction and reported differences in the folate content of dry bean among some market classes but not between cultivars in the same market class. Location had a significant effect on the folate content of lentil and dry pea; cultivar did not. The significant effect of market class, cultivar, and growth environment on the levels of folate in pulses is of particular importance to pulse breeders [26]. Reported that folate concentration in lentil cultivars ranged from 216 to 290 μg/100 g with a mean of 255 μg/100 g and a significant year x location interaction for lentil folate concentration has been reported. A small red lentil cultivar, CDC Rouleau, showed the highest concentration of 290 μg/100g and a large green cultivar, CDC Greenland, showed the lowest (216 μg/100g) concentration of folate. In addition, they have also shown that lentil showed higher folate concentration as compared to chickpea (range 42-125 μg/100 g; average of 65 μg/100 g), yellow field pea (range 41-55 μg/100 g; average of 50 μg/100g), and green field pea (range 50-202 μg/100 g; average 105 μg/100g). Further they have also reported that a 100 g serving of lentils could provide a significant amount of the recommended daily allowance of dietary folates (54-73%), whereas 100 g of serving of yellow field pea, green field pea, and chickpea can supply 12%, 26%, and 16% of the daily folate intake requirement for adult, respectively [44]. Determined the concentration of folates in four cultivars each of common bean, lentil, chickpea and pea, and also studied the effect of growing location on folate concentration. In this study, six folate monoglutamates were quantified by ultra-performance liquid chromatography coupled with mass spectrometry (UPLC-MS/MS).
Total folate concentration ranged from 351 to 589 mg/100 g in chickpea, 165 to 232 mg/100 g in common bean, 136 to 182 mg/100 g in lentil, and 23 to 30 mg/100 g in pea. The 5-methyltetrahydrofolate (5-MTHF) and 5-formyltetrahydrofolate (5-FTHF) folates were most abundant in common bean, lentil and chickpea, whereas 5-MTHF and tetrahydrofolate (THF) were the predominant forms in pea. Significant differences were detected among cultivars for all folates across the pulses, except for 5,10-methenyltetrahydrofolate (5,10-MTHF) in lentil, 5-MTHF in chickpea, and 5,10-MTHF and folic acid (FA) in pea. Significant effects for location and cultivar by location were also observed for the majority of the folates [45]. studied the genetic variability for folate in lentil cultivars and reported significant genotypic difference in the lentil genotypes (p < 0.1) for folate content which was estimated using the trienzyme extraction and high-performance liquid chromatography method with an average value of 222 μg/100 g lentil dry seed weight.
The range of variability for folate content was 114 ± 3 to 330 ± 7 Hg/100 g dry seed weight. The mean folate content of Mediterranean landraces of lentil was higher (247 μg/100 g lentil dry seed weight) as compared to the other genotypes (198 μg/100 g lentil dry seed weight), respectively. Folate Content of Legumes were determined by microbiological (Lactobacillus casei subsp. Rhamnosus ATCC 7469) and high-performance liquid chromatography analysis utilizing a tri-enzyme treatment (protease, a-amylase and conjugase) [46]. Folate derivatives of tetrahydrofolate, 5-formyl-tetrahydrofolate and 5-methyl-tetrahydrofolate were identified and quantified. The total folate content of cooked legumes ranged from 53 to 81 μg/100 g for beans; 133 to 203 μg/100 g for peas, and from 39 to 22 μg/100 g for small and large lentils, respectively. The predominant form of folate in legumes was tetrahydrofolate, followed by 5-formyl- tetrahydrofolate and 5-methyl-tetrahydrofolate.
Malnutrition is one of the world's largest health concerns. Folate (also known as vitamin B9) is essential in the human diet, and without adequate folate intake, several serious health concerns, such as congenital birth defects and an increased risk of stroke and heart disease, can occur. Most staple food crops, are poor sources of dietary folates, however, legumes have traditionally been considered as a good dietary source of folates. Economically, legumes (Fabaceae) represent the second most important family of crop plants after the grass family, Poaceae. Grain legumes account for 27% of world crop production and provide 33% of the dietary protein consumed by humans, while pasture and forage legumes provide vital part of animal feed. The genetic and trait diversity available to a crop underpins any attempt at genetic improvement. That trait diversity may come from elite cultivars, from unimproved landraces or even from related species. This chapter discusses about the genetic variability of folate content in major legumes and the potential for genetic biofortification in these crops.
The author is thankful to ICAR-Indian Institute of Pulses Research, Kanpur, UP, India for financial assistance in the form of an in-house research project for conducting studies on the related areas and also wish to extend thanks to Dr. N. P. Singh, Director, ICAR-Indian Institute of Pulses Research, Kanpur, UP, India for allowing to use the research facility at the institute to conduct the related study.


