Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ken Yaegaki*
Received: March 29, 2018; Published: April 09, 2018
*Corresponding author: Ken Yaegaki, Department of Oral health, Nippon Dental University,1-9-20 Fujimi, Chyodaku, Tokyo, Japan
DOI: 10.26717/BJSTR.2018.03.000934
H2S has been shown to act as a biologically active compound in mammalian cells; H2S may also involve cardio protective or cardiovascular therapeutic effects. The concentrations of NaHS, used instead of H2S at - mM, in most of previous studies are higher than the IC50 or Ki of cytochrome c oxidase (COX) activity by H2S. However we found COX is inhibited by only 500 nM H2S, reactive oxygen species causing DNA double-strand breaks are produced, and the mitochondrial membrane is depolarized. Following the above redox reactions, the p53 pathway is activated. Consequently, apoptosis is initiated. If the lowest concentration of H2S (1 nM) is applied for hepatic or pancreatic differentiation from human-tooth pulp, the differentiation or proliferation is heavily promoted through WNT signaling and PI3K-AKT signaling pathways. A possibility of regenerative medicine or reversal ageing using H2S at nM level is also suggested. On the other hand previous studies clearly indicated that the accuracy of dose-response studies using NaHS or Na2S at ^M - mM are questionable, we cannot produce constant concentration of H2S using NaHS. NaHS is easily vaporized, and the dissociation constant of H2S is not equal to that of NaHS. This presents a huge discrepancy affecting investigations of redox biology. The concentration of H2S used for in vitro or in vivo experiments is strongly recommended to be determined by a precise and suitable measure. The review focuses on effects of H2S on apoptosis, typical de-differentiation process, differentiation of the stem cells, and regenerative medicine.
Keywords: Hydrogen sulfide; Biologically-active compound; Reactive oxygen species; Apoptosis; Stem cell; Tooth
Abbreviations: H2S: Hydrogen Sulfide; COX: Cytochrome C-Oxidase; MDA: Malon-di-Aldehyde; ROS: Reactive Oxygen Species; CBS: Cystathionine P-Synthase ; CSE: Cystathionine y-Lyase; DCFH-DA: Di-Chloro-Fluorescein Diacetate; HGF: Human Gingival Fibroblasts; Caspase: Cysteinyl- Aspartic-Acid-Proteases; NO: Nitric Oxide; CO:Carbon Monoxide; MAPK: Mitogen-Activated Protein Kinase; ERK: Extracellular Signal-Regulated Kinase ; IPS: Induced Pluripotent Stem; ES: Embryonic Stem
Hydrogen sulfide (H2S) is a poison gas like cyanide; both are strong inhibitors of cytochrome c oxidase (COX; EC 1.9.3.1), which is a crucial enzyme for the respiratory chain in mitochondria [13]. Thus, H2S readily causes cell suffocation leading to death; people attempt suicide using home-produced H2S [4]. H2S causes human death rapidly but the IC50, half maximal (50%) inhibitory concentration of COX activity caused by H2S in animals is 0.13 μM-0. 30 mM [5-7]. H2S has been also shown to be a gas transmitter, meaning that H2S is one of the biologically-active compounds required for initiating or supporting biochemical processes in mammalian cells [8]. Gas transmitters are able to enter into the cell. Cell membranes present no barrier to them [9]. This means that H2S is unlike many of the regular transmitters, which need receptors on the cell membrane to control the signal transduction systems in the cells [8]. The barrier for H2S permeation on the Gibbs energy profile is negligible, and the hydrophobicity of H2S allows it to get into the paraffinic interior of the membrane freely [10]. H2S might control or regulate the process more easily than the regular transmitters do, since H2S permeates cell membranes so easily [10]. Thus, H2S at low concentrations may be involved in many biological activities in mammalian cells.
There is some doubt about the H2S concentrations utilized in studies determining the biological activity of H2S, if H2S is provided by exogenous NaHS or Na2S. Most of the studies employed 501000 μM of NaHS in place of H2S for their in vitro studies [11-13]. Some have even employed Na2S instead of H2S. The concentrations utilized in these studies are much higher than the IC50 of COX activity by H2S. Even animal studies employed higher concentration of NaHS than the IC50 determined by [5]. Therefore, cell toxicity of H2S is expected, but the studies have been reported only the positive activities of H2S rather than its cytotoxicity. In measuring malondialdehyde (MDA) content [14] found that NaHS at 100 μM, which is higher than the IC50 determined by [5], almost completely scavenged 0.3% H2O2 oxidative stress. Other studies employing NaHS as exogenous H2S have demonstrated that a high concentration of H2S of over 50 μM, also higher than the IC50, reduces oxidative stress in mammalian cell culture in which reactive oxygen species (ROS) were increased by chemical or other pathological interventions [13,15-19]. On the other hand, no changes were found in cells having a healthy level of ROS (without any intervention) after exposure to high concentrations of NaHS [16,19]. Hence, anti-oxidative activity of 50 μMH2S is uncertain; the H2S concentration in these reports may not be same as active free H2S concentration. However, antioxidant activity by H2S has been strongly suggested in mammalian tissues. H2S endogenously produced by cysteine reduces ROS, but its concentration is not clear [20].
The usefulness of NaHS in treating cardiac disorders, especially myocardial injury, has been reported [8,13-19,21-24], many studies utilized much higher concentration of H2S than the IC50. It has been clearly indicated the potential ability of H2S to control or prevent these conditions, although convinced concentrations of free active H2S has not yet determined. Very high concentration of NaHS at the mM level causes COX inhibition followed by depolarization of mitochondrial membrane [12], leading to the production of large amounts of ROS. Such a concentration of H2S produces oxidative stress through ROS production [11]. This speculation seems to be correct, since a H2S concentration higher than that causing anti- oxidative activities increases oxidative stress as reported by others [8,11,13-15]. These results were supported by [25]. It has been claimed that most tissues contain H2S at the concentration range of approximately 30- 300 μM [15]. However, the cells will not be able to survivein the mM level of H2S.
There is a further contradiction here: such a high concentration of H2S at 30-300 μM is higher than the IC50 of COX activity by NaHS [5]. Moreover, the Ki, the inhibitor constant, of H2S to COX is only 0.2 μM [26] reported that the real concentration of H2S is only around 15 nM in many tissues, less than one thousandth of the concentration previously claimed, although they spent much time for sampling: this means the production of extra H2S may happen. Butthey used a precise gas chromatograph with flame photometric or chemiluminescence detector to determine H2S concentration, moreover they produced gas samples for H2S determination rather than tissue-fluid samples containing much non-free H2S. So [5] and [27] detected only the free form of H2S. Furthermore amount of H2S produced by both NaHS and Na2S is not constant. H2S production rate depends on pH [28]. Hence, as [5] did, a gas chromatograph measurement is needed to find the exact concentration of H2S. Further discussion regarding the inconsistency of H2S concentration in the tissues may be required.
Besides NaHS vaporizes quickly. Although a reliable sensor with perfect specificity to H2S has not yet developed [29,30], found much faster loss of H2S with using a semiconductor sensor, and reported their inability to maintain H2S concentration; also half time, the time required to lose half of the H2S, is only 5 min or less in several experimental conditions: they concluded that the accuracy of dose- response studies are questionable. Part of the basis for the above comments is that a stock solution of NaHS utilized for the H2S studies is never recommended for H2S studies [14]. On the other hand [31] have reported that NaHS causes dilatation of human pulmonary arteries and attenuates cardiac dysfunction. This fact is undisputed; however, the concentrations of H2S actually utilized in those experiments might be different from those reported, because of the inability to determine the true concentration of NaHS present [32].The method of [33] is frequently employed to determine H2S concentration [8,19,34,35]. However the method of [33] quantifies the concentration of inorganic sulfide including H2S, the method is not specific to detect H2S. A problem lies in that they used NaHS, but they were unable to determine how much of the free form of H2S was present before [5,26] or [29] described how to measure concentrations of H2S in the free form with using a gas chromatography with a flame photometric detector [29,36] and [37] determined the certain concentration of H2S effecting on several tissues with using a H2S gas generator producing a constant concentration instead of NaHS (Figure 1), and the effects of H2S at a nM level on cell metabolism, the respiratory chain and on apoptosis have been determined. The role of H2S during de-differentiation is relatively well investigated as mentioned above.
Figure 1: H2S gas-producing system for keeping a constant concentration. NaHS instead of H2S gas is utilized for most other studies, but these do not determine the concentration of the free form of H2S in the medium. As NaHS is easily gasfied, there must be a large loss of SH. Using both our system and a gas chromatograph we can produce less than 100 nM H2S constantly. Instead of the PermeaterTM (GASTEC, Kanagawa, Japan), the Dynacalibrator (VICI Metronic, Houston, USA) can also be uti.
On the other hand, information on its role during differentiation is very limited. H2S exhibits an important role in the cell viability through controlling redox homeostasis by cysteine/ glutathione metabolism including cystathionine p-synthase (CBS) and Cystathionine y-lyase (CSE) [13,38,39], and by mediation through ion channels [40]. As well, activation of the PI3K-Akt pathway does not inhibit apoptosis However, the relationship between H2S and stem-cell differentiation nor the role of H2S during differentiation has been clearly determined. The efficacy of the gas transmitter H2S in regenerative medicine using human-tooth-pulp stem cells has been determined, and a clear positive effect on cell differentiation in regenerated tissues was found [37,41-43]. The role of H2S during differentiation is also discussed in this review.
It has been previously reported that high concentrations, around 100 μM of NaHS, increased ROS scavenging activities in mammalian cells [8,13-15,20]. The following hypothesis is still under discussion: H2S evokes a strong antioxidant activity against ROS in mammalian cells, consequently reducing oxidative stress. It has also been suggested that d-cysteine may protect tissues, especially heart, from ROS and even from ischemia, due to H2S produced from d-cysteine by CBS, CSE or 3-metacaptopyruvate sulfurtransferase [8,13-15,20]. Hence [13] have been implied that H2S decreases the amount of pathologically-elevated ROS by inhibiting the ROS-activated NF-kB pathway or by increasing the activity of superoxide dismutase (SOD) [15]. Hence, pathologically elevated ROS might be decreased by administrating NaHS as an exogenous form of H2S [15, 19], although the real concentration of active H2S is not clear.
On the other hand, NaHS does not increase ROS in mammalian cells that are in a normal or healthy condition [19]. Based on those studies there is another hypothesis: it is claimed that H2S administration might be a novel cardiovascular therapeutic procedure [8,16]. The assertion that such a high concentration (hundred μM level) NaHS can protect the cells from ROS rather than causing damage to the cells may need to be taken under careful reconsideration and further research for the following reasons: [44] reported that a 100 μM sulfur introduced by H2S inhalation causes human death. Cysteine, around 100 μM, is known to cause cell death, and cysteine significantly elevates in patients who suffer early stroke deterioration, cysteine converts to H2S, indicating that H2S may be acting as a mediator of ischemic brain damage [45].
Inhibition of H2S formation was also suggested as a novel approach in acute stroke therapy [45,46] implied a cause of sudden infant death syndrome (SIDS): the H2S detoxifying system at the colon surface may not have matured by the age of 3 months, so H2S could possibly be absorbed, resulting in SIDS. It is clear that H2S inhibits COX, leading to cellular asphyxia [1,47,48]. In the mitochondrial respiratory chain COX is the terminal enzyme (complex IV), SIDS might increases ROS production dramatically in a burst [48,50,51]. There is another outstanding question concerning the protective or curative effect of NaHS in situations like heart or myocardial conditions, i.e. both pathways of AMPK and AKT are activated by H2S for cardio protection, but AKT negatively controls AMPK [19,49,52]. These contradictions might happen because they did not make accurate and exact measurements of active H2S concentration [32].
To confirm the hypothesis that H2S has protective/treatment effects on heart conditions, further studies are required to determine the safe and effective concentration of H2S that will prevent or treat these conditions. On the other hand, application of 500 nM H2S, which is less than that found in human gingival crevicular fluid in periodontal conditions producing much amount of volatile sulfur compounds as the colon does [53], and which is less than one hundredth of the exogenous NaHS utilized in previous researches, increased the amount of ROS, including all mitochondrial ROS present, and inhibited SOD activity [54], which results are totally different from those found by other researchers [11,13-15,55]. However, all of them used μM level or more of NaHS [11,13,15] utilized 2',7'-dichlorofluorescein diacetate (DCFH- DA) to determine ROS value. DCFH-DA detects mainly hydrogen peroxide or hydroxyl and peroxyl radical in cytosol even though the manufacturer's instructions claim that the product is for detecting ROS [14,56] detected malondialdehyde which is byproduct from ROS, they did not directly measure ROS [55] did not determined amount of O2-. But [54] detected O2- in mitochondria.
Furthermore H2S at 500 nM does not increase any activity of ROS scavengers [36,54,57-61]. On the other hand 500 nM H2S inhibited SOD strongly and increased ROS greatly [54]. If such a low concentration 500 nM of H2S inhibits SOD and produces ROS, much higher H2S concentrations (μM levels) may not be able to scavenge ROS. The effects of exogenous NaHS on ROS production and the process are not clear. CuZn-SOD (E.C.1.15.1.1; Sigma-Aldrich, Oakville, ON, Canada), Mn-SOD (E.C.1.15.1.1; Wako Pure Chemical, Tokyo, Japan) and also SOD in human gingival fibroblasts (HGF) homogenate were exposed to 500 nM H2S [54]. The percentage inhibition of CuZn-SOD and Mn-SOD was found to be around 7080 % for 1h incubation and increased in proportion to incubation time. The inhibition of HGF-SOD was 80% [54]. They found that such a low concentration of H2S inhibits SOD activity rather than protecting the cells from ROS. All cells in their studies are human cells. It is very unlikely that a concentration as high as 50-100 μM of NaHS would reduce oxidative stress.
There is a possible ironic explanation of why 50-100 μM of NaHS reduces ROS: since a high concentration of NaHS reduces cell vitality, biological products including ROS might also decrease. Part of the toxicity of H2S to mammalian cells is ROS production, which may cause DNA damage [11,12,62]. Further studies would be required to confirm if H2S increase or decreases oxidative stress.
Gasotransmitters that regulate physiological responses are produced endogenously, but extraneously-produced H2S also affects the human tissues. Human oral tissues are much easily and simply biopsied compared with other human tissues, and can be a source of human-cell primary cultures. Moreover it is possible to obtain a large number of volunteer, each volunteer gives one independent result. The effects of extrinsic H2S on several fresh tissues or cells from humans have been easily determined [36,54,57-61]. Their hypothesis is that H2S is the cause of oral conditions such asperiodontitis and ageing of mucosa or alveolar bone, since they used the concentration of H2S existing in human gingival crevices, and as the tissues are exposed to H2S for one or more day. Ng and Tonzetich [63] reported that the permeability of human mucosa is dramatically increased after exposing the epithelial surface to H2S; around 100% of the H2S penetrates and gets through the epithelia and into the underlying tissues.
The effects of penetrating H2S on biological transduction may escalate to pathological reactions, resulting in damage to the integrity of the tissue [54] concluded that H2S causes DNA damage by increased ROS. DNA damage caused by ROS is at the root of apoptosis caused by intrinsic pathways to protect the individual from expected conditions, e.g. malignant tumors [64]. Many papers have reported or suggested that high concentrations of exogenous H2vS reduce oxidative stress; however, the effect of increased H2S on DNA has not yet been made clear [8,13-15,20,64] found that 50 μM NaHS caused micronuclei formation, an indicator of DNA damage using human lung fibroblasts [54] and others [36,57-61] found or suggested DNA damage caused by only 500 nM H2S in several human tissues or cells. H2S concentration was determined by a gas chromatography with flame photometric detector as described above. H2S is expected to cause DNA damage followed by activating the p53-pathway for apoptosis [64].
DNA damage was evaluated by single-cell electrophoresis (Comet AssayTM TTevigen) [64] utilized DNA ladder agarose electrophoresis for confirming DNA damage. However the purpose of DNA ladder is for detecting apoptosis. But Comet AssayTM was designed to detect DNA damage. The basis of Comet AssayTM is the electrophoretic migration of DNA in an agarose gel. Intact or relatively-intact DNA migrates very slowly and stays within the nucleus, but damaged DNA migrates quickly. More damaged DNA, i. e. more short fragmented DNA, and migrates much faster. Thus, the end-product has the appearance of a comet comprised of a head, which is the nuclear region containing healthier DNA, and a tail containing DNA fragments. To evaluate DNA damage, comet-tail shape is assessed. There are many protocols to evaluate tail shape, but tail length, percentage of DNA in the tail and tail moment were frequently measured [65].
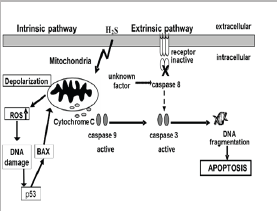
A significant difference between test (exposure to H2S) and control (non-exposure to H2S) groups was found with the Comet AssayTM , measuring tail length, tail moment and DNA in tail; tail length especially was significantly increased in proportion to incubation time [55]. It has been clearly demonstrated that H2S at 500 nM causes DNA damage [64] indicated that H2S evokes the caspase (cysteinyl-aspartic-acid-proteases) pathway in human lung fibroblasts, because H2S may cause DNA double strand breaks. In the apoptotic process involving mitochondria, caspase-3 is an executing enzyme [66]. In proportion to oxidative stress and DNA damage, caspase-3 activity is increased [54,60]. They assessed the mitochondrial membrane potential treated with 500 nM H2S. Under H2S exposure for 1-3 days around 25% of the cells were depolarized, while the control group showed only 5 - 6% depolarized cells.
Releasing cytochrome c from mitochondria into cytosol is an important process in the activation of the caspase pathway. They detected increased cytochrome c release from mitochondria, and determined that cytochrome c activates caspase-9. The released cytochrome c increased around 10 times compared with controls [60]. They also measured the activities of caspase-9 and -8, finding that only caspase-9 was increased by H2S [60]. Also, in the cells originated from several human tissues, only caspase-9 was activated [36,57,61]. However [67] reported that H2S increased both caspase-8 and -9 in osteoblasts. Hence they investigated the effects of H2S on the receptors and functions of the extrinsic caspase pathway: human Fas ligand, TNF-a, IL1-a, IL-b, IL-2, IL- 4, IL-10, interferon-c, granulocyte-colony-stimulating factor and granulocyte macrophage-colony-stimulating factor. None of these receptors or cytokines was activated by H2S, suggesting that novel factor, not yet been determined, may stimulate caspase-8 [67]. From the above findings, the apoptotic pathway initiated by 500 nM H2S is summarized as shown in (Figure 2).
Figure 2: Simple apoptotic pathways caused by H2S. Broken line indicates activity in osteoblasts [36].
H2S is a gasotransmitter like nitric oxide (NO) or carbon monoxide (CO). NO and CO cause or inhibit apoptosis, depending on cell types [68]. There are arguments on both sides as to whether H2S inhibits or causes apoptosis [17,25,64,68], reported the anti- apoptotic effect of 100 μM H2S in cardiomyocytes by antioxidative activity of H2S through the SIRT1 pathway [17] found that NaHS at 500 μM induces cell proliferative or anti-apoptotic effects by activating the NF-KB pathway. But 100- 500 μM of NaHS was utilized as the exogenous H2S sources in these studies are much higher than IC50 or Ki. On the other hand [68] investigated whether H2S caused apoptosis in human aorta smooth-muscle cells. Exogenous H2S originated from NaHS did not induce significant cell necrosis and LDH release, but very high concentration NaHS (500 μM) increased the number of condensed apoptotic nuclei, accompanied by the typical morphological changes of apoptosis.
Furthermore, NaHS also increased the number of TUNEL- positive cells, and DNA fragmentation was confirmed by DNA ladder agarose gel electrophoresis. NaHS induced apoptosis in human aorta smooth-muscle cells. They found around 10% incidence of apoptosis after incubating the cells with 200 μM NaHS for 12 h. Also similar apoptosis happened in neurons [69,70] carried out cell-cycle analysis after exposure to H2S expected to be lower than 1μM, using the same H2S-exposure system as [54] used (Figure 1).
The proportion of cells in G(1) phase was significantly increased and the cells in S phase significantly decreased. Moreover Rb phosphorylation was reduced and p21(Cip1) expression was increased after exposure to H2S. They concluded that H2S inhibits cell proliferation and induces cell-cycle arrest through p21(Cip1) in the HeLa cell line. It was also suggested that H2S might cause apoptosis in human-oral keratinocytes [36,54,57,58,61] found that a similar apoptotic process determined in the Hela cells also happened in human-skin keratinocyte stem cells and human primary oral keratinocyte stem cells. Human oral-keratinocyte stem cells were separated from human biopsies using a protocol established by [58]. p53 expression was induced by 500 nM H2S in the cells [57]. As previously demonstrated, the number of apoptotic cells among these cells was significantly increased after exposure to 500 nM H2S [57-59].
In the apoptotic process DNA fragmentation indicating apoptosis was increased, ROS production was also increased, mitochondrial membrane potential was collapsed, and there was a significant amount of cytochrome c released into the cytosol. Caspase-9 and -3 activities were also significantly increased by H2S exposure, but caspase-8 activity was not increased except osteoblasts MC3T3-E1 from mouse [57,58,67] measured p53 and the related gene BAX to determine if the p53 pathway is stimulated by DNA damage caused by 500 nM H2S. A member of the Bcl-2 protein family, Bax is the primary response gene to p53, and starts after p53-mediated apoptosis [71]. Total p53, phosphorylated p53 Serine 46 and Bax were significantly increased after H2S incubation. In particular, both total and p53 phosphorylated p53 at serine 46 were dramatically increased after exposure to H2S. These data demonstrated that the p53 pathway plays the most important role in apoptosis caused by H2S. A simple apoptotic pathway caused by 500 nM H2S is summarized in (Figure 2). The expression of all genes appearing in the p53 pathway activated by 500 nM H2S was determined; more details of the p53-pathway-related cell cycle and DNA repair are found (Figure 3) [61]. From these reports it was suggested that the gasotransmitter H2S, of which the concentration might be much less than previously expected, may cause apoptosis.
Figure 3: Cell cycle, DNA repair and apoptotic pathways initiated by 500 nM H2S [61].

[49] found that 100 μM NaSH inhibited mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase p38 pathways, and then activated PI3K/Akt signaling in cardiomyocytes, when H2S reduced high-glucose-induced apoptosis both in vitro and in vivo. On the other hand [72] found that 500 nM H2S inhibits the proliferation of osteoblasts through the MAPK pathway as shown in (Figure 4). This contradiction might depend on the differences in the concentrations or the tissues. It was also found that 500 nM H2S causes apoptosis in osteoblasts, as described above [37,67] determined the effects of H2S on osteoclast differentiation. When the murine macrophage cell line RAW264 was exposed to only 1 nM H2S instead of 500 nM, osteoclasts were differentiated from the cell without nuclear factor-kappa B ligand (RANKL) [37].
Figure 4: H,S controls the proliferation of human osteoblasts through MAPK pathway [72].

A recent review has demonstrated that the strength of osteoclast differentiation by a receptor-activator of RANKL is dependent on the redox state of the precursor cells, macrophages, that is controlled by H2S [73], [37] determined the effects of MAPK inhibitors on RAW264 cell differentiation by H2S, extracellular signal-regulated kinase (ERK) inhibitor and p38 inhibitor suppressed osteoclast differentiation by H2S. They concluded that H2S at 1 nM in the medium differentiated osteoclasts from RAW264 through MAPK [37,74] determined the effects of NaHS on nicotine- and LPS-induced osteoblastic and osteoclastic differentiation, and found that the differentiations were inhibited by MKP-1 enzyme inhibitor (vanadate) and expression inhibitor (triptolide). These findings strongly suggest that H2S has effects on the differentiation of stem cells, especially adult stem cells.
H2S influences a number of biological compounds, one of them being T-type Ca2+ channels. The T-type Ca2+ channel is the low- voltage activated channel activated by depolarization of the plasma membrane. The T-type Ca2+ channel involves three isoforms: Cav3.1, Cav3.2 and Cav3.3. H2S appears to affect Cav3.2 channel activity especially using human embryonic kidney cells [75]. Cav3.2 channel is blocked by zinc, since it binds to a histidine residue on the channel protein [76]. H2S activates Cav3.2 channel by interacting with zinc binding to the histidine residue at the channel [77]. H2S also activates the KATP channel through cysteine sulfhydration, resulting in relaxation of vascular muscle [40]. Neurite outgrowth and altered electrophysiological changes, i.e. an increase of voltage- gated Na+ and/or Ca2+ channels, in neuronal progenitor or stem cells indicate neuronal differentiation of the progenitor or stem cells [78]. Very high concentrations of H2S (mM level of NaHS), which would not exist in mammalian cells, induced neuronal differentiation from the progenitor or stem cells, since neurite outgrowth and increase of the high voltage-activated current in the cells through the channels were observed [79]. Consequently, activating Cav3.2 T-type Ca2+ channels with H2S induces neuronal differentiation of the progenitor cells.
The relationship between H2S and neuronal differentiation was also brought into focus [40] but this has not yet been done for other tissues. It has been suggested that endogenous H2S protects neurons from oxidative stress [38]. Oxidative stress is a plausible reason for neuronal damage or conditions in the brain, including stroke, epilepsy, and Alzheimer's disease [80,81]. Oxytosis, oxidative stress caused by glutamate, is a typical condition for neural tissues. H2S protects primary neuron cultures of rat cortex from cell death caused by oxytosis [38]. Glutathione is produced by enhancing the activity of γ- glutamylcysteine synthetase and up-regulating cysteine transport. Cysteine is the substrate of glutathione synthesis. Glutathione normally decreases oxidative stress caused by H2O2. Glutamate inhibits transport of cysteine into the cell, thus oxytosis is caused by glutamate. They found that NaSH at 100 μM increases the glutathione concentration [38]. In this way H2S protects neurons from oxytosis.
Moreover [82] reported that H2S protected neuronal cells from formaldehyde-induced oxidative stress through the BDNF- TrkB pathway, reducing oxidative stress and controlling apoptosis by increasing Bax expression and decreasing Bcl-2 expression. Because of the anti-oxidative activities of H2S, a positive effect on differentiation from neural stem cells can be expected [81] examined the effects of H2S on mouse neural stem cells, and the underlying molecular mechanisms. NaHS at 0.5 - 5μM promoted proliferation and neuronal differentiation of neural stem cells, NaHS-induced proliferation was caused by activating ERK. Neuronal proliferation was also activated through expression of the basic helix-loop-helix transcription Factors: neurogenin 1, NeuroD2 and mammalian achaete-scute homologue 1 [81,83,84] reported that NaHS increased the proliferation of human iPS-cell-derived mesenchymal stromal cells via the PI3K-Akt pathway [21].
Also determined the effects and mechanism of endogenous H2S on the in vitro proliferation and differentiation of neural stem cells, They found that L-cysteine stimulated proliferation and increased the differentiation potential of neural stem cells through cystathionine β synthase/ H2S and ERK pathways. In an in vivo study, NaHS administration increased the number of proliferating cells in the hippocampus of mouse after hypoxia. Moreover the administration of NaHS allowed recovery from cognitive impairment in mice subjected to hypoxia. Hence, a H2S donor could be used for neuronal treatment, and to improve endogenous neurogenesis [81].
It has been shown that transplantation of hepatocyte-like cells differentiated from human tooth pulp perfectively treated liver cirrhosis produced in rats [85]. It was never happened before, although adult stem cells are being used to treat diseases. On the other hand, embryonic stem (ES) cells and induced pluripotent stem (iPS) cells demonstrate the largest capacity for multilineage differentiation. However, the former involves an ineluctable ethical problem and the great possibility of producing a malignant tumor. The latter may have the same problems, and genetic modification might cause further trouble in the future: its possibility of producing malignancy may be higher than in adult stem cells [86]. In fact. Institute of Physical and Chemical Research (Japan) send-off their second retina transplantation using human iPS cells because of DNA sequence modification [87].
Regenerative medicine using stromal mesenchymal stem cells is much safer than using ES or iPS cells. Human-tooth pulp cells showed expression of CD117, nanog, nestin, CD44, alkaline phosphatase, Oct3/4, CK18, CK19, osteonectin and P63. Nanog and Oct3/4 are markers of ES or iPS cells [88]. Approximately 70% of the primary culture showed stem-cell markers, and the CD117+ fraction, which forms 50% of the primary culture, is now utilized for hepatic or pancreatic differentiation. The protocol for hepatic differentiation directly from deciduous- and wisdom-tooth pulp was established [88], and then a new protocol using non-serum medium for hepatic differentiation was developed [89]. It was also suggested that H2S may directly affect hepatic and pancreatic differentiation [37,41-43].
On the other hand the relationship between H2S and human- tooth-pulp stem cells has also been well reported. The CD117+ fraction of deciduous-tooth-pulp stem cells were incubated with 1 nM H2S produced using a PermeaterTM (GASTEC, Kanagawa, Japan), as shown in (Figure 4). All the cells differentiated into hepatic cells [41]. Glycogen storage happens in hepatocyte-like-cells; it is the distinguishing function of hepatocytes. Increased accumulations of glycogen were shown in the cells exposed to 1 nM H2S after several days (Figure 5). Urea concentrations in the medium of the cells increased after H2S exposure [41]. It was concluded that H2S exposure produces more matured hepatocyte-like cells which have much less possibly of producing malignancy compared with initialized stem cells or direct transplantation of stromal stem cells without in vitro differentiation. They have also compared the effects of H2S exposure on human deciduous-tooth-pulp stem cells with those on human-bone marrow stem cells [42,43].
They confirmed that the expression level of stem-cell-related transcription factors (112 factors) was almost the same between CD117+ tooth-pulp stem cells and bone-marrow stem cells in six groups: ectodermal-lineage markers, mesodermal-lineage markers, endodermal-lineage markers, stem-cell/embryonic- development markers related to late embryonic development, axis/ symmetry/segmentation-markers associated with early embryonic development, and other pluripotency markers supporting pluripotency and regenerative abilities. DLX2 and MSX2 showed significant increases in tooth-pulp stem cells compared with bone- marrow stem cells; significant decreases were found in EGR3 and NANOG. Expression of OLIG2, GATA6, EGR3 and ESR1 was found only in bone-marrow stem cells [43].
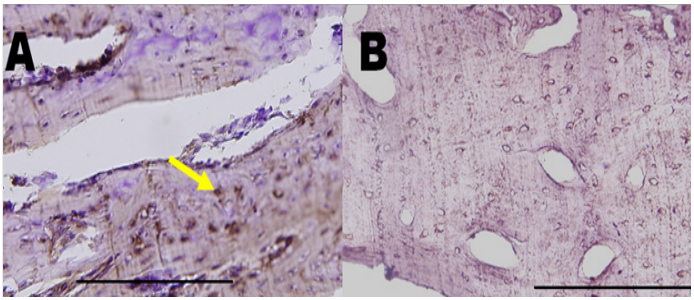
Figure 5: Glycogen storage in hepatocyte-like cells. (A) Cytoplasmic glycogen storage after the differentiation period (PAS staining, *200). (B). The area was evaluated with Image J, and the proportion of positive cells in samples exposed to H2S was compared with that in non-exposed samples [41]. *p<0.05, **p<0.001.
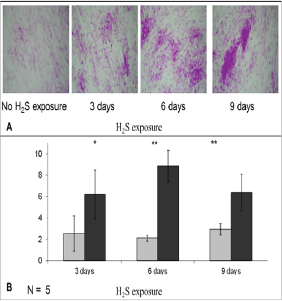
Figure 6: Flow cytometric analysis. Almost 100% of cells were positive to ALB, a- FP and CPS by microscopic examination; however, flow cytometric data showed smaller numbers, possibly because of a higher detection threshold. It was expected that more matured cells would be detected by a flow cytometer. Significant differences amongst CD117+ tooth-pulp stem cells, bone-marrow stem cells and H2S treatment were found [42]. *p < 0.01.
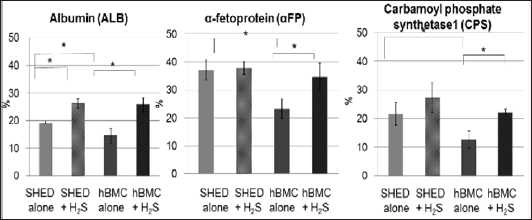
Almost 100% of hepatic differentiated cells were positive for albumin, a-fetoprotein and Carbamoyl phosphate synthetase1 by microscopic examination, hence quantitative flow-cytometric data was obtained and showed smaller numbers than those obtained by microscopic examination, perhaps because of the higher detection threshold (Figure 6). It had been anticipated that more matured cells would be detected by a flow cytometer. Human-tooth showed more positive cells for albumin, a-fetoprotein and carbamoyl phosphate synthetase! than human-bone-marrow stem cells. H2S increased the number of positive cells in both types (Figure 6) [42]. Concentration of urea in the culture medium was determined after hepatic differentiation. Urea concentration for H2S-treated cells significantly increased in the media of both cell types, compared with non-H2S-treated control cells. H2S increased urea concentrations in a time-dependent manner. After 9 days of H2S exposure, the urea concentration had increased almost one-and-a-half fold compared to non-H2S-treated cells. Urea production in non-H2S-treated tooth pulp was also found to be significantly higher than non-H2S-treated bone-marrow cells. It was concluded that H2S promotes hepatic differentiation from human-tooth pulp stem cells, and thus more matured hepatocyte-like cells are obtained. Hepatocyte-like cells differentiated under exposure to H2S might result in great clinical success when these cells are utilized to regenerate the liver in future. As described above, even implantation of non-H2S-treated cells was able to treat liver cirrhosis produced in rats (Figures 7 & 8) [85], but no preclinical study using hepatocyte-like cells has succeeded before. H2S-treated cells might be able to transform the regenerated liver into mature [90]. Differentiated pancreas-like cells from the CD117+ fraction of human-tooth-pulp, describing the pathways of pancreatic transcription factors and hormones in differentiating the cells into pancreatic cells (Figure 9) they also determined the effects of H2S on pancreatic differentiation from human-tooth, and how H2S affects it through WNT and P13K-AKT signaling pathways [43].
Figure 7: Treatment of liver cirrhosis in rats using regenerated hepatic cells originating from human tooth pulp. Cirrhosis nontransplanted demonstrated the typical results of blood examinations from cirrhotic rats, whereas cirrhosis transplanted, which had received transplantation of hepatocyte-like cells originating from human tooth cells, showed similar results to the control. More than half of the albumin was human albumin [85].
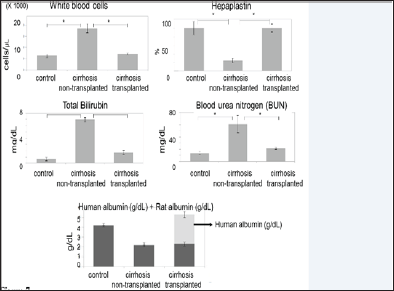
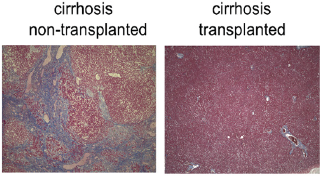
Figure 8: Treatment of liver cirrhosis in rats using regenerated hepatic cells originating from human tooth pulp. Cirrhosis non-transplanted demonstrated typical cirrhosis pathology, but fibrosis had disappeared in cirrhosis transplanted, which had received transplantation of hepatocyte-like cells originating from human tooth cells [85]. Masson's trichrome stain. Magnification 200*.
Flow-cytometric analysis for cells expressing insulin and glucagon was carried out. H2S increased the number of cells expressing insulin compared to non-H2S-treated cells, but decreased the number of glucagon-positive cells. Thus the pancreatic cells produced by H2S are appropriate to treat diabetes mellitus. To understand the relationship between H2S and insulin or pancreas, the excellent review by Wang [91] is useful. He cited [92,93] and Patel and Shah [94], and concluded that H2S regulates insulin secretion from pancreatic p cells by enhancing the KATP channel and suppressing L-type Ca2+ channel activities. KATP channels are activated by H2S at concentrations, which is more than the IC50 of COX [9,92-95] wrote a review of the regulation of mitochondrial bioenergetic function by H2S. Interestingly [96] described protective activities of H2S on pancreatic p cells. Insulin concentration in pancreatic cells or medium was determined when applying glucose stimulation (Figure 10) [43].
Figure 9: Pathways of pancreatic transcription factors and hormones. Analysis was performed by Ingenuity Pathway Analysis. FGF1, EGF and HGF utilized for the pancreatic differentiation protocol of human dental pulp activate the pancreatic transcription factors PDX1, HHEX, NEUROG3, PAX6, NKX6-1, Pax-4 and MNX1, and result in production of pancreatic hormones. Solid lines represent direct interactions. Dashed lines represent transduction pathways involving different and diverse cytoplasmic mediators activating the transcription factors in the nucleus. PPY: pancreatic polypeptide, SST: somatostatin, GCG: glucagon and INS: insulin [90].
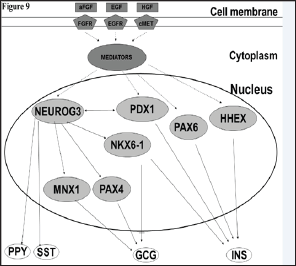
Figure 10: Glucose stimulation of pancreatic cells originating from human tooth pulp. Without glucose stimulation H,S increased insulin concentration. Glucose at 3.3 mM increased insulin concentration in both the medium and the cells, and also in both the H2S+ and H2S- groups. However, 16.7mM glucose stimulation decreased insulin concentration compared with 3.3 mM glucose.
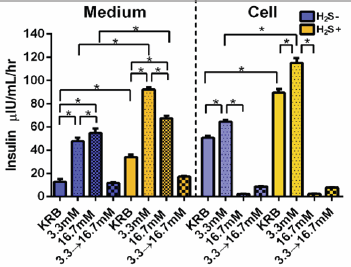
Exposure of the cells to H2S increased intracellular or extracellular insulin concentration under no glucose stimulation. Glucose at 3.3mM increased insulin concentration in both the medium and the cells, and also in both the H2S-treated and nontreated groups. However, stimulation with 16.7mM glucose decreased insulin production compared with 3.3 mM glucose, except in the medium without H2S exposure. Moreover, 16.7mM glucose stimulation following 3.3 mM glucose decreased insulin concentration greatly (Figure 10). However, the amount of C-peptide inside the cells showed the highest value in those treated with 16.7mM glucose stimulation following 3.3mM glucose under H2S exposure. This means that when the first stimulation with 3.3 mM was carried out, the cell was already exhausted [43]. On the other hand it was found their pancreas-like p cell has same function for insulin secretion as shown in the cells derived from human embryonic stem cells [97].
WNT signaling is mainly composed of the canonical pathway, the planar-cell polarity pathway, and a calcium-ion-dependent pathway. All these pathways were activated by H2S but expression of WNT-signaling negative-regulation genes was very weak [43]. H2S increased the level of expression in PI3K-AKT-related genes [43]. This pathway involves the following: AKT and PI3K family members and their regulators, the IGF-1 signaling pathway, the BAD phosphorylation and anti-apoptotic pathways, the inactivation of Gsk3 and accumulation of p-Catenin, and PTEN-dependent cell- cycle arrest and apoptosis. Genes involved in all functions of the PI3K-AKT signaling pathway were significantly expressed after H2S exposure compared with the non-treated group [43]. It was elucidated that H2S promotes the differentiation from human- tooth pulp through WNT signaling and PI3K-AKT pathways. The data using both 1 nM of H2S and adult stem cells showed that lower concentration of H2S strongly promotes differentiation of adult stem cells, a possibility of reversal ageing using H2S, e.g. H2S releasing NSAIDs, is suggested.
H2S promotes ROS production, DNA damage and p53- mediated apoptosis at much lower concentrations than previously reported. The lowest concentration of H2S promotes both hepatic and pancreatic differentiation of human-tooth pulp stem cells. The real concentration of H2S is only around nM level in human tissues, less than one thousandth of the concentration previously claimed. Moreover less than one- thousandth or hundredth of the concentration previously reported showed the toxicities causing apoptosis in many tissues. Low concentration such as 1 nM promotes hepatic and pancreatic differentiation from human tooth through WNT signaling and PI3K-AKT pathways.
The author thanks Drs. Ishikawa H, Nakahara T, Imai T, Kumazawa Y, Tanaka T and his previous and present PhD students for their excellent work.


