Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Goichi Matsumoto*1,2, Yoshihiko Sugita2, Katsutoshi Kubo2, Waka Yoshida2, Hatsuhiko Maeda2, Mamoru Aizawa3, Shigetaka Shimodaira4 and Yukihiko Kinoshita1,2
Received: March 30, 2018; Published: April 09, 2018
*Corresponding author: Goichi Matsumoto, Department of Oral and Maxillofacial Surgery, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Kahoku-gunn, Ishikawa 920-0293, Japan
DOI: 10.26717/BJSTR.2018.03.000931
Aim of this study was to show that modified calcium phosphate cement (CPC) that includes gelatin particles effectively promotes bone regeneration in the canine mandible. The modified CPC is a mixture of hydroxyapatite (HAp) particles, whose surfaces have been modified with inositol hexaphosphate (IP6), and a -tricalcium phosphate (α -TCP) powder Mixing powdered gelatin with the HAp surface-modified with IP6 / α -TCP cement creates a composite that becomes porous after setting in vivo. For this experiment we produced four experimental mixtures, each with a different concentration of gelatin particles. We created bone defects in the mandibles of beagle dogs, and we injected one of the four experiment materials in each defect. After 6 months, mandibles were removed for micro-CT and histological analyses. These analyses showed that at experimental sites at which the cement had a gelatin particle mixing ratio of 0 mass% and 5 mass%, new bone had formed only at contact boundaries of unresorbed cement. In contrast, at each experimental site at which the gelatin particle mixing ratio of the cement was 10 mass% or 15 mass%, the cement specimen was almost completely resorbed and new bone had formed at the peripheral and internal areas of the original cement mass.
Keywords: Chelate-setting calcium phosphate cement; Inositol hexaphosphate; Gelatin particles; Alveolar bone regeneration; Canine mandible
Calcium phosphate cement (CPC), hydraulic cement composed of hydroxyapatite (HAp), is one of the bone substitutes that have been used to treat compression fractures of the hip and vertebrae [1-3]. It is produced by combining a powder containing calcium and/or phosphate with a liquid phase to form a paste that hardens in a few minutes. Before setting, CPC paste is easily shaped and can be directly injected or pressed into a bone defect during a surgical procedure. It is rigid enough to retain its shape and position, and it can be contoured so that it replaces the lost bone and restores its original shape. The cement setting reaction is a dissolution and precipitation process, and the entanglement of the precipitated crystals is the mechanism responsible for cement hardening [4]. Research has led to modifications of pure CPC that make the new materials better for clinical use. For example, the pH of pure CPC after it sets causes inflammation of the tissues surrounding it [5]. Thus, we developed a mixture of materials (HAp surfaced modified by IP6; hereafter, IP6-HAp) in which the setting reaction is a chelating reaction rather than an acid-base reaction. Our studies showed that IP6-HAp is as biocompatible as pure CPC, i.e., that the in vitro growth rate of osteoblast-like cells cultured on IP6-HAp cement is similar to that on HAp cement [6,7]. Our studies also showed that if we added α-TCP powder as a component of the cement paste, it would quicken the setting time and increase its anti-washout capability. We further improved our new material mixture by adding 1.0% citric acid to a mixing solution containing chondroitin sulfate to obtain greater strength of the hardened cement. The mixture of IP6-HAp, α -TCP, chondroitin sulfate, and citric acid forms the basis of our present research to further improve this cement [8]. After solving the inflammation problem, decreasing the setting time, and obtaining greater strength of the material, we are left with one major hurdle to overcome. The in vivo resorption rate of conventional injectable CPC is very slow [9]. Because CPC is more fragile than bone, an oral implant site remains susceptible to fracture under loading until the cement is resorbed and replaced by new bone. This jeopardizes the successful clinical outcome of an oral-implant placement.The slow resorption rate of CPC is due to its dense structure. It is not porous enough to allow bone-marrow cells and other cells that generate new bone to penetrate into the interior of the material [10]. Like CPC, our modified IP6-HAp / a -TCP cement lacks pores of sufficient size to allow the infiltration of cells. Investigators have shown that CPC that contains an interconnected network of pores permits cells to migrate into its interior. These cells build bone throughout the mass of cement [11-13]. Introducing gelatin particles into CPC is one way to create the interconnected pores. The gelatin particles dissolve quickly in vivo, opening up the pores. In a previous study, we showed the usefulness of this kind of injectable CPC that contains gelatin particles to facilitate the healing of defects in the alveolar ridge of the mandible [14]. The composites we have developed are more biodegradable and more osteoconductive than pure CPC, and they are therefore more clinically useful than pure CPC. In view of these considerations, the goal of the current study is to investigate the effectiveness of injectable IP6-HAp / α-TCP cement that contains gelatin particles in healing surgically created defects in the alveolar ridge of the canine mandible.
A sodium inositol hexaphosphate (IP6-Na) solution at a concentration of 8,000 ppm was prepared using 50 mass% phytic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and adjusted to a pH of 7.3 with NaOH solution. Ten grams of commercially available HAp powder (HAp-100; Taihei Chemical Industrial Co. Ltd., Osaka, Japan) was simultaneously ground and surface-modified by ball-milling with 50 cm3 of the 8,000 ppm IP6-Na solution, using a planetary ball-mill for 60 min at a rotation rate of 300 rpm in a ZrO2 pot with 180 g of ZrO2 beads 2 mm in diameter. After ball-milling, the resulting slurry was filtered and freeze-dried for 24 h to yield the IP6-HAp powder [8].
We ground 1,200 grams of commercially available α-TCP raw powder (α-TCP; Taihei Chemical Industrial Co., Ltd., Osaka, Japan) for 120 min at a rotation rate of 300 rpm, using a ball mill in an alumina pot with 11.9 kg of ZrO2 beads 3 mm in diameter and 6 g of ethanol. After ball-milling, the slurry mixtures were filtered and freeze-dried for 24 h to obtain a -TCP powder.
The commercially available powdered gelatin used in this study was extracted from porcine skin, alkaline-processed (isoelectric point of 5.0, Nitta Gelatin Co. Ltd., Osaka, Japan), and cross-linked by heat at 140°C for 14 hours under vacuum conditions. Gelatin particles had irregular shapes. The size distribution of particles was broad, in the range 5 to 666 μ m, and the median particle size was 184 μ m.
The mixing solution contained 15 mass% sodium chondroitin sulfate, 2.5 mass% disodium hydrogen phosphates, 1.0 mass% citric acid, 0.076 mass% sodium hydrogen sulfite, and 81.424 mass% distilled water. It was adjusted to a pH of 7.0 with NaOH solution. All the chemicals were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan.
The IP6-HAp powder was premixed with the prepared α -TCP powder at a mixing ratio of 20/80 (g/g). The experimental design called for testing four graft materials (Gl0, Gl5, Gl10, and Gl15) that were formed with four different mixing ratios of IP6-HAp / α -TCP cement powder and gelatin particles. The mixing ratio of gelatin particles in these materials was 0 mass%, 5 mass%, 10 mass%, and 15 mass%, respectively. The balance of the material (100 mass%, 95 mass%, 90 mass%, and 85 mass%, respectively) was IP6-HAp / α -TCP cement powder. We mixed 2g powder with 1.2 ml of liquid to produce the Gl0, Gl5, Gl10, and Gl15 materials. The IP6-HAp / α -TCP cement powder, gelatin particles, and liquid were sterilized before combining to make the cement paste.
Morphology of the prepared gelatin particles was observed by a scanning electron microscope (SEM) (TM- 10000 Tabletop Microscope, Hitachi, Tokyo, Japan).
Four one-year-old female beagle dogs, approximate 10 kg body weight were used for the experiment. The Animal Care Committee of Aichi Gakuin University authorized this study. The animals had access to a standard laboratory diet and water until the beginning of the study.
Treatment procedures have been described previously [14]. Briefly, all animals were under general anesthesia (32 mg/kg pentobarbital sodium (Kyoritsu Seiyaku, Tokyo, Japan) and dental infiltration anesthesia (1.8 ml of 2% lidocaine hydrochloride and 1:80000 epinephrine) was used at the surgical sites. We extracted all first molars and all second, third, and fourth premolars on both sides of the mandible to create space for identical bone defects three months before treatment. A symmetric pattern of four saddle-type defects was created in the left and right edentulous alveolar ridges of the mandible in all animals. The bone defects (mesiodistal length of 10 mm and depth of 5 mm) were outlined with a dental bur in a standard handpiece while being irrigated with sterile saline. The mesial and distal bone defects were separated by 4-5mm (Figure 1). In each animal, each of the four bone defects was immediately filled with a cement paste made from one of the four experimental materials using a 2.5 ml syringe. The paste was shaped to recreate the pre-existing edentulous alveolar ridge (Figure 1) and the mucoperiosteal flap was returned to its natural position and sutured with 4-0 Vicryl (Ethicon, Inc., USA). All animals received an intra-muscular injection of broad-spectrum antibiotics (Viccillin, Meiji Co., Tokyo, Japan, 50mg/kg/day) on each of the first three postoperative days. They were fed a soft diet for the first month. Every animal was clinically observed at least once a week to monitor the mucosal health and the state of wound closure. All experimental defect sites healed without infectious symptoms (swelling, pus discharge, etc.) during the six-month postoperative healing period.
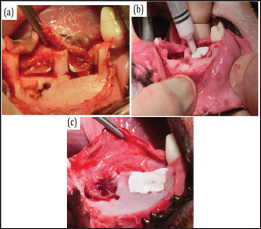
Figure 1: Clinical appearance of the surgically created bone defects with injected IP6-HAp / α -TCP cement and gelatin composite. Intraoperative view of two saddle-type bone defects created in the mandible (a). Bone defects were filled with IP6-HAp / α -TCP cement paste using a syringe (b and c).
At six months after placing the graft materials, we gave the animals an overdose of pentobarbital sodium and removed their mandibles. They were placed in a solution of 70% ethanol for 5 days after which the soft tissue around the mandible was removed. Bone samples were positioned on the stage of a micro-CT instrument so that the shaft was oriented vertically. The X-ray generator was operated at an accelerating potential of 90 kV with a beam current of 50 ^ A using a Micro X-ray CT System (R-mCT, Rigaku Mechatronics, Tokyo, Japan). Each slice consisted of 480 x 480 pixels. Slice thickness was 0.8 mm. Mimics software (Mimics 14.0, Materialise, NV, USA) was used to reconstruct images on a personal computer. The volume of interest of the bone in the defect site was calculated. The bone volume and bone density of the reconstructed alveolar ridge were measured using Mimics software. To determine the relative bone density of newly generated bone, it was normalized with neighboring normal cortical bone [14].
Procedures have been described previous report [14]. The bone samples were sectioned perpendicularly to the axis of the middle portion of each defect site. The non-decalcified blocks were dehydrated serially in three concentrations of ethanol (80, 90 and 100 vol%; 2 days at each concentration) and then embedded in LR White resin (Okenshoji Co., Ltd. Tokyo, Japan). Specimens 20 μm thick were sectioned using a band saw perpendicular to the bucco- lingual plane. Surfaces of the sections were polished with diamond paper, subjected to Villanueva bone staining, and observed under a light microscope. For histomorphometric analysis, an image- analysis system (HC-2500/OL; Win ROOF; Mitani Co., Japan) was used to quantify the area of unresorbed IP6-HAp / α -TCP cement based on manually traced contours. The image-analysis software determined the area of unresorbed cement in cross-sections. This area was converted to percent by dividing by the area of the reconstructed alveolar ridge.
For each animal, we calculated the difference between the volumes of bone in the alveolar ridge at each experimental site. P values were computed with Student's t-test. A p-value less than 0.05 were considered statistically significant.
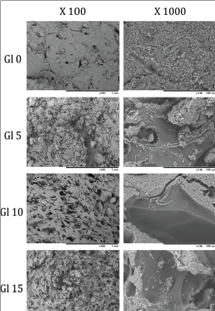
Figure 2 shows the morphology of each of the four IP6-HAp / α -TCP cement composites, each containing a different concentration of gelatin particles. All four preparations appeared well-preserved. Gl0 contained only small gas bubbles without micropores. The surface of the cement exhibited a smooth appearance. On the other hand, Gl5, Gl10, and Gl15 contained large gelatin particles that gave the surface of the cement a relatively rough appearance and that, when resorbed, would open up micropores.
Figure 2: Scanning electron microscope (SEM) images of the morphology of pure IP6-HAp / α -TCP cement (Gl0) and those of three mixtures of IP6-HAp / α-TCP cement and gelatin powders (Gl5, Gl10, and Gl15). These three mixtures contain 5 mass%, 10 mass%, and 15 mass% gelatin, respectively, with the balance of the material IP6-HAp / α -TCP cement (magnification 100x and 1000x).
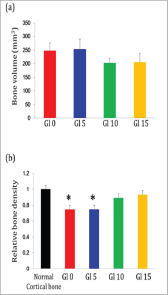
By six months after surgery the alveolar ridge at all experimental sites had been restored to its preoperative contour. In Gl0 and Gl5 sites, injected IP6-HAp / α -TCP cement was still detectable as regions having relatively low radiopacity. The boundary between bone and residual IP6-HAp / α -TCP cement was clearly distinguishable, and the areas surrounding unresorbed IP6-HAp / α-TCP cement had relatively high radiopacity. In contrast, unresorbed IP6-HAp / α -TCP cement was not clearly observed in Gl10 and Gl15 sites, and the alveolar ridges of these defect sites were completely replaced by bone. The border between the bone adjacent to the surgical site and the new bone that had formed within the surgical site was almost undetectable. On the other hand, the vertical height of the alveolar ridges of experimental sites was apparently less for Gl10 and Gl15 sites than for Gl0 and Gl5 sites (Figure 3a). We reconstructed 3D micro-CT images in order to compute the volume of the newly formed alveolar ridge. The volume of the alveolar ridge at Gl10 and Gl15 sites was less, but not significantly so, than at Gl0 or the Gl5 sites. The volume of the alveolar ridge at Gl10 sites was not significantly different than at Gl15 sites (Figures 3b &4a). Next, we measured the density of the newly formed alveolar bone. The bone density of the newly formed alveolar ridge at Gl0 and Gl5 sites was significantly lower than that at Gl10 and Gl15 sites, and it was significantly lower than neighboring normal cortical bone. On the other hand, the mean bone density of new bone at Gl10 and Gl15 sites was not significantly different than the density of the neighboring normal cortical bone (Figure 4b).
Figure 3: Micro-CT images of the frontal sections of mandibular bone defects at Gl0, Gl5, G110, and Gl15 sites six months after surgery. Newly generated alveolar bone in each treatment group is shown with dotted squares (a). Three-dimensional micro-CT reconstructed appearance of bone regeneration in the mandibular bone defects six months after surgery (b). B, buccal side; L, lingual side.
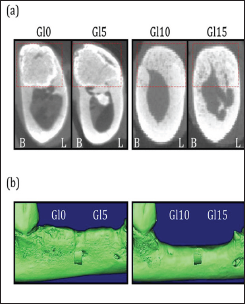
Figure 4: Graph of newly generated alveolar bone volume and density as determined by three-dimensional micro- CT analysis. The volume of new bone at six months after surgery (a). The relative bone density of new bone at six months after surgery. Data were normalized with the density of the neighboring normal cortical bone. *P<0.05 versus Gl10, Gl15, and normal cortical bone.
Low-magnification images of the Gl0 and Gl5 sites showed that resorption of IP6-HAp / α-TCP cement was limited to the periphery of the injected cement. Newly formed mature bone was observed only at the periphery of unresorbed regions of IP6-HAp / α -TCP cement. There was no further in growth into the IP6-HAp / α -TCP cement, corroborating the micro-CT results. Bone had started to form wherever the IP6-HAp / α -TCP cement intimately contacted bone. The augmented radiopaque regions showed where cement had been completely replaced by bone tissue. Far more IP6-HAp / α -TCP cement had been resorbed at the periphery of Gl0 and Gl5 healing sites than in their interior regions. At the interface between bone and IP6-HAp / α-TCP cement, no fibrous tissue was observed. Much less IP6-HAp / α -TCP cement was observable at Gl10 and Gl15 sites than at Gl0 and Gl5 sites.
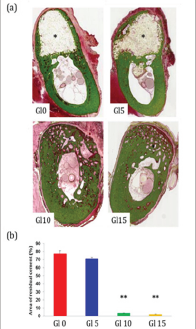
The contour of the original alveolar ridge had been almost completely restored with mature bone tissue (Figure 5a). Histomorphometrical analysis showed that the area of residual IP6- HAp / α-TCP cement at G5 sites (73.2 ±1.9%) was approximately the same as at Gl0 sites (77.2 ±3.8%). The area of residual IP6- HAp / α-TCP cement at Gl10 and Gl15 sites was 3.8 ±0.4% and 2.3 ±0.2%, respectively (Figure 5b). Gl10 and Gl15 sites showed accelerated IP6-HAp / α -TCP cement resorption compared to the other two groups. The residual IP6-HAp / α -TCP cement at Gl10 and Gl15 sites resembled isolated islands that were completely surrounded by newly generated bone. A photomicrograph at higher magnification shows that osteoclasts were resorbing IP6-HAp / α -TCP cement at all sites and osteoblasts were forming new bone in the remodeling lacunae at Gl10 and Gl15 sites. Gl10 and Gl15 sites demonstrated speedy remodeling, including IP6-HAp / α -TCP cement resorption and lamellar bone formation.
Figure 5: Histological microphotographs at six months after surgery. Villanueva bone staining: Gl0, Gl5, G110, and G115. Newly formed bone was observed at the periphery of the unresorbed IP6-HAp / α -TCP cement (*). Regenerated calcified lamellar bone was not observed in the interior or at the periphery of the unresorbed cement (Gl0 and Gl5). The IP6-HAp / α -TCP cement (*) graft had been almost completely resorbed and replaced by new bone during the healing period. Note the asymmetric alveolar ridge contour generated by new bone (Gl10 and Gl15) (a). Area of unresorbed IP6-HAp / α -TCP cement injected in dog mandible at six months after surgery (**P<0.01 versus Gl0 and Gl5) (b).
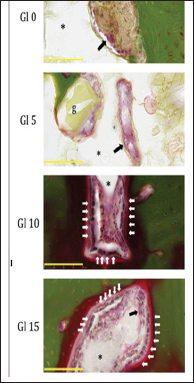
At Gl10 and Gl15 sites, we identified osteoclasts in the remodeling lacunae of the porous IP6-HAp / α -TCP cement. More osteoblasts were present and more new bone was forming at these sites than at Gl0 and Gl5 sites. Osteoblasts were observed along the edges of new bone. None of the experimental sites contained any inflammatory cells at the junction between IP6-HAp / α -TCP cement and bone (Figure 6).
Much has already been established about the in vivo resorption characteristics and bone formation of IP6-HAp / α -TCP cement. Studies of surgically created defects in the tibia of rabbits showed that 100% IP6-HAp / α -TCP cement was not resorbed and replaced by new bone [6,7]. In another case report with IP6-HAp / α-TCP cement hybridized with gelatin particles (10 mass%) injected into bone defects in the tibia of pigs, the experimental material was resorbed within 8 weeks and newly formed bone was identified in the internal and peripheral areas of the residual cement [15]. Our present study results confirm that for grafts of 100% IP6-HAp / α -TCP cement (Gl0) the processes of resorption and new bone formation take place almost exclusively at the periphery of the implanted cement. Like the earlier investigators, we observed some interior portions of grafts of 100% IP6-HAp / α -TCP cement still present long after the IP6-HAp / α -TCP cement was implanted. In a previous experiment we showed that in both Gl10 and Gl15 cements the regions where implanted cement had been resorbed extended into the interior of the grafts. Based on this result, we chose gelatin particle mixing ratios of 0 wt%, 5 wt%, 10 wt% and 15 wt% for our present experiment. Here we have shown that at Gl0 and Gl5 sites, resorption of IP6-HAp / α -TCP cement was limited to the periphery of the implanted material. Newly formed bone was observed only at the periphery of implanted material and was absent in its interior. In contrast, at Gl10 and Gl15 sites, resorption of all regions of the implanted IP6-HAp / α -TCP cement was so extensive that the original implanted mass was almost unrecognizable. The micro-CT morphometric analysis showed that the bone volume at Gl10 and Gl15 sites was less than at Gl0 and Gl5 sites. However, at the G0 and G5 sites, bone density of newly formed bone, as determined by micro-CT, was statistically lower than that of normal cortical bone.
Figure 6: Higher magnification of the IP6-HAp / α -TCP cement specimens with Villanueva bone staining: Gl0, Gl5, Gl10, and Gl15. Black arrows indicate osteoclasts. Osteoclasts were found at the resorbing front where the IP6-HAp / α -TCP cement was being resorbed and new bone was taking its place. Osteoblasts (white arrows) are behind new bone and around the surface of new bone. A large number of osteoblasts were found around the surface of new bone in the remodeling lacunae at Gl10 and Gl15 sites. IP6-HAp / α -TCP cement (*) and gelatin particles (g) are present. (bar = 100 (μm).
These results imply that the large amount of unresorbed graft material at Gl0 and Gl5 sites was responsible for the lower bone density. The mean volume of the regenerated alveolar ridge for Gl10 and Gl15 grafts at the end of the experiment suggests that the grafted material shrinks over an unknown time period and that shrinkage is related to the concentration of gelatin microparticles in the graft mixture. Histological observation demonstrated that while the Gl0 and Gl5 specimens were relatively stable due to their dense structures and new bones were formed only in the peripheral area, the Gl10 and Gl15 specimens were almost resorbed by macroporosity formation and subsequent migration of cells, allowing the new bone formation in the internal area. The presence of gelatin particles in IP6-HAp / α -TCP cement tends to increase its setting time and reduce its mechanical strength [16]. The gelatin hybridization extended the initial setting time of the cement paste, resulting in the washout, and dramatically decreased the compressive strength. This is probably because the gelatin particles disturbed entanglement of the precipitated crystals of HAp and decreased the relative density of the specimen [15]. We have offset these disadvantages by adding a polysaccharide, chondroitin sulfate, and by adding citric acid. The inclusion of these components promotes the hardening reaction and increases the mechanical strength of IP6-HAp / α -TCP cement. In fact, we demonstrated that the addition of 15 mass% sodium chondroitin sulfate and 1.0 mass% citric acid could modify the material properties of the gelatin particles in IP6-HAp / α-TCP cement [17-19]. As gelatin particles in IP6-HAp / α -TCP cement is easy to handle and could quickly enhance bone regeneration without cell transplantation and harvesting of autogenous bone for grafting, clinical application of gelatin particles in IP6-HAp / α -TCP cement as a cost-effective bone regenerative material for patients. We have made much progress in refining and modifying CPC to make cements that work well to restore bone defects. In spite of our progress, it is clear that further preclinical studies as well as further clinical trials will be necessary to ascertain the most advantageous combination of these variables.
The present study demonstrates that an injectable material made of IP6-HAp / α -TCP cement mixed with gelatin particles has good handling properties and effectively regenerates new bone in mandibular bone defects in dogs. More importantly, it is resorbed quickly enough in vivo to be of clinical use to augment the healing of alveolar-ridge defects in humans. Toward this end, the resorption characteristics of mixtures of IP6-HAp / α -TCP cement and gelatin particles should be further investigated. Based on our results, we conclude that gelatin-hybridized IP6-HAp / α-TCP cement injected into bone defects is resorbable under in vivo conditions and has good biocompatibility. This study confirms the suitability of the material as an alveolar bone replacement in bone defects.


