Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ibrahim Rudhani1, Naim Morina*2 and Ahmet Avdullahu3
Received: February 28, 2018; Published: March 21, 2018
*Corresponding author: Naim Morina, Resident of Internal Medicine Clinic of Nephrology and Hemodialysis UCCK Kosovo Hospital Circle n.n. 10000 Pristina, Kosovo
DOI: 10.26717/BJSTR.2018.03.000877
The purpose of this study was to evaluate the effect of Pentoxifylline on the reduction of proteinuria of type 2 diabetes mellitus patients who were being treated with ACE and ARB. The study was prospective and was achieved for six months at the Nephrology Clinic, UCCK, between January and June 2016. The study included 162 patients with type 2 diabetes, diabetic patients with proteinuria above 500 mg / 24h despite being treated with ACE Inhibitors and ARB. The patient group is treated with Pentoxiphylline (oral) 400 mg 3 times a day. Proteinuria for 24 hours was evaluated at the beginning of the study, then in the first quarter and after six months treatment with Pentoxiphylline. In the other group 80 healthy individuals treated with Placebo. Both groups have adapted the study to eliminating patients with comorbidities. To the all patients and subjects of the control group were taken anamnesis, also it was made objective examination, biochemical laboratory tests and hematologic tests of Proteinuria for 24 hours. The standard deviation and T-test are used for data analysis. During the six-month study of these patients treated with Pentoxiphyllin 400 mg (oral) proteinuria was very early. In the first three months, there was a decrease in proteinuria or decreased protein loss through urine. The average age was 59.13, of which 86 males and 76 females were involved in the research.
Keywords: Diabetes Mellitus; Nephropathia Diabetica; Pentoxifylline; Chronic Kidney Failure
Diabetes mellitus is a group of common metabolic disorders characterized by increased glycemic blood levels. With the increasing prevalence of diabetes worldwide, it still remains one of the most common causes of morbidity and mortality in the world. Although the prevalence of both types of diabetes is increasing worldwide, it has been expected that type 2 diabetes mellitus (T2DM) will grow more in the future due to obesity and unpleasant physical activity. Late (secondary) complications to diabetics are: retinopathy, neuropathy and nephropathy. Diabetic nephropathy is considered the most common cause of kidney failure (ESRD) [1]. Recently, treatment options have been increased in decreasing disease progression by improving kidney function.Approximately 35 to 50% of all ESRD-dependent (kidney disorder) need active treatment (replacement of kidney function), including Hemodialysis, peritoneal dialysis or abdominal transplant are complications of diabetes type 2 diabetic nephropathy. Since long-range dialysis is costly and causes disabilities to work for the patient, the preventive not to advance in the stages of kidney failure or ESRD seems to be the most logical way to reduce patients' costsand suffering and also postpone active dialysis treatment or kidney transplantation [2].
In major studies done in a large number of patients with diabetes, proteinuria is the worst being a risk factor for advancement at the last stage of renal disease [3]. Increased risk for kidney damage justifies the need to reduce Proteinuria in these patients [4]. Currently ACEs and ARBs are used to reduce Proteinuria [5]. Therefore, maximum renal protection can be reached by decreasing proteinuria [6]. In almost all cases, more than one treatment is needed to achieve this goal [7]. Recent research has shown that pentoxifylline (PTX) has the ability to reduce proteinuria in diabetic patients with normal renal function [8]. Pentoxifylline is a methylxanthine which regulates blood flow and a non-selective phosphodiesterase inhibitor which reduces inflammatory factors including TNF-, IL-1 and IL-6 and plays an important role in the pathogenesis of interstitial renal fibrosis and advancing diabetic nephropathy [9-11]. Existing experimental human and animal data show that there is a strong scientific basis for the use of pentoxifylline as an antiproteinuric medicament [12,13].
For this reason, it appears that pentoxifylline can maintain kidney function with further reduction of proteinuria and then reduces cardiovascular complications. The first line therapy in preventing or decreasing the loss of protein in the urine is ACE or ARB which usually is achieved a satisfactory modification but in other cases the use of Pentoxifylline, which is satisfying, decreases the proteinuria for 24 hours and also other complications resulting from protein loss [14]. The cardiovascular mortality rate in these patients is 10-20 times higher than the total population [15]. Arterial hypertension occurs in 60-90% of patients with renal insufficiency, prior to hemodialysis [16]. Congestive heart failure is a cause of mortality at around 20-30% of cases in these patients (2.4).
The purpose of this study was to evaluate the efficacy of pentoxifylline in reducing proteinuria in patients with type 2 diabetes mellitus although they were being treated with ACE and ARB.
The study was conducted at the Hemodialysis Nephrology Clinic, University Clinical Center of Kosovo, from January 1, 2016 to June 30, 2016. The study was prospective including 162 patients with type 2 diabetes, diabetic patients with proteinuria above 500 mg/24 h, despite being treated with ACE inhibitors and ARB. The patient group is treated with Pentoxifylline 400 mg 3 times a day. Proteinuria for 24 hours was evaluated at the beginning of the study, then in the first quarter and after six months. The other group included 80 healthy subjects treated with Placebo. Both groups have adapted to the study, patients with comorbidities have been eliminated. To the all patients and subjects of the control group were taken anamnesis, and objective examination was made, biochemical laboratory tests and hematologic tests of Proteinuria for 24 hours. The statistical methods of the application are descriptive analysis (standard deviation averages), and T test.
Out of 162 patients in the study treated for six months with Pen- toxiphyllin 400 mg (oral), three times a day by 1 tablet, proteinuria was very early in the first three months that there was a decrease in the values of Proteinuria or decrease of protein loss for urine. The average age of patients was 59.13 (Figure 1). Out of 162 examined and treated patients, there were 86 males and 76 females (Figure2). At the beginning of the treatment, the measurable proteinuria values were 2.64 g per 24 hours at a maximum of 5.7 g / l. 24 hours, minimum 0.9 g / l for 24 hours. After three months of treatment with tab. Pentoxifylline 400 mg, a tablet three times a day, the average proteinuria value is 1.50 g / l, maximum is 4.1 g / l and minimum 0.56 g / l for 24 hours (Figure 3). After 6 months treatment with tab therapy Pentoxifylline 400 mg, a tablet three times a day the average proteinuria value was 1.16 g / l, the maximum value is 2.2 g / l and the minimal value is 0.22 g / l (Figure 4). In the study group with placebo there were 37 females and 43 males, the average age of respondents in this study was 63.69 years. Initially proteinuria was 125 mg, in the second quarter was 145 mg, and at six months it was also 125 mg, which is at the limit of normal proteinuria (Figure 5).
Figure 1: Graphic presentation of the most attacked age by diabetic Nephropathy.
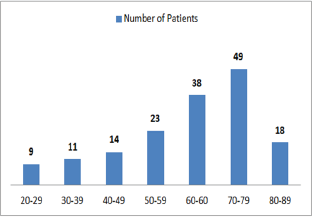
Figure 2: Representation of the Sex Ratio between Diabetic Nephropathy.
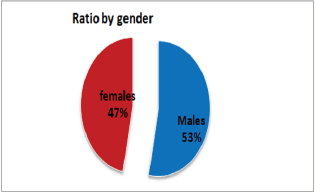
Figure 3: Graphic presentation of proteinuria after treat-ment with Pentoxifylline of diabetic nephropathy.
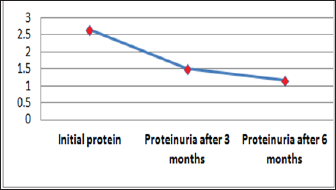
Figure 4: Graphic presentation of proteinuria after treat-ment with Pentoxifylline of diabetic nephropathy
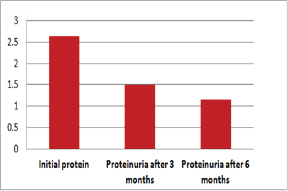
Figure 5: Presentation of proteinuria in control group (placebo) patients.
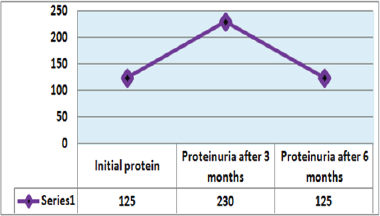
From the examinations of our study we can see that men dominate more. Out of 162 patients treated, there were 86 males and 76 females while the average age of patients was 59.13. At the beginning of treatment with tab. Pentoxiphylline 400 mg, a tablet three times a day, since the beginning there is decrease in proteinuria value. After three months of treatment with tab. Pentoxifylline 400 mg, a tablet three times a day at maximum starting point of 5.7 g / l. 24 hours, there is a decrease after three months therapy and the value was reduced to 1.50 g/ l. After 6 months of treatment with tab. Pentoxifylline 400 mg, a tablet three times a day the average proteinuria value was 1.16 g / l, the maximum value is 2.2 g / l and the minimal value is 0.22 g / l. In the study group with placebo consisting of 80 subjects from whom 37 were female and 43 males, the average age of this subject was 63.69. At first, Proteinuria was 125 mg, the second quarter also 145 mg and the half year was also 125 mg which is at the limit of normal proteinuria [6].
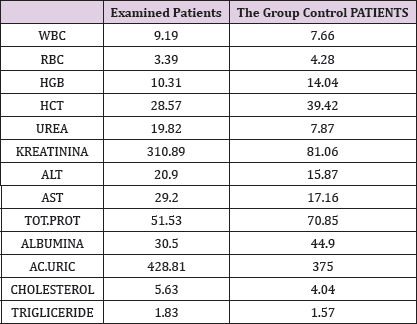
In the framework of our study, we conducted the empirical analysis through the T-test, comparing the values of laboratory analysis in the group of patients treated with Pentoxifylline 400 mg a tablet three times a day to the placebo patients group including three periods. From the results of the analysis we see that in three periods the proteinuria values in patients treated with therapy compared to the group of patients without therapy are significant because the value of P is 0,000 which means it is smaller than the allowed value p = 0.05. Comparison of leukocyte values between traumatic patients and control group also shows a significant difference since the P value is p = 0.002 which means that it is less than p = 0.05. Leukocyte lab analysis values are also presented in Table 1. Based on the results, a significant difference is observed in the comparison of erythrocytes in patients treated with therapy and control group as the value p = 0.033, which means that it is 0.05. The values of the laboratory analysis of erythrocytes are also presented in Table1 and Figure 6. Unlike the leukocyte values and erythrocytes, hemoglobin values in patients treated with Pentoxiphyllin 400 mg there aren't any significant difference with placebo patients. The values of the laboratory analysis of HGB are also presented in Table1 and Figure 6.
Figure 6: Graphic presentation of the values of erythrocytes and hemoglobin in patients with diabetic nephropathy and pa-tients in the control group.
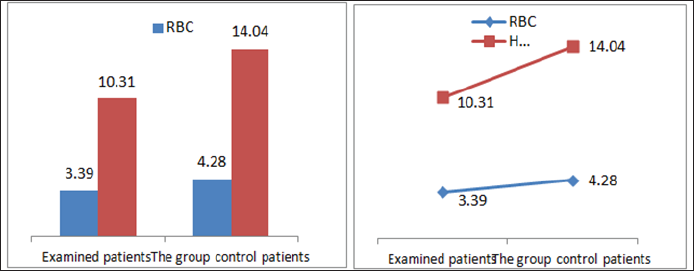
Table 1: Presentation of the analyzed values from examined patients and the patients of the control group.
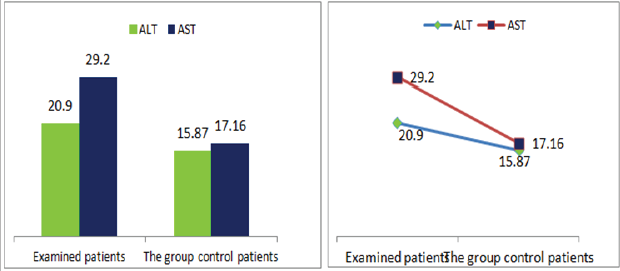
Also a significant difference based on the results is the values of Hematocrit, Creatinine, total of proteins, uric acid between treatments and control groups. This significance is confirmed by the value of P (HCT p-Values = 0.000, Creatinine p-Value = 0.000, Total Proteins p-value = 0.002 and Uric Acid p-Value = 0.000) where in four cases is less than 0.05. These values are also presented graphically (Figures 7 & 8). While the values of laboratory analysis that did not have any significant statistical difference between patients treated with Pentoxiphyllin 400 mg and placebo patients were: transaminase values (ALT, P-Value = 0.382), AST values (AST, P-Value = 0.483), albumin values (albumin, P-Value = 0.603), cholesterol values (Cholesterol, P-Value = 0.063) and triglyceride values (Triglycerides, P-Value = 0.062). In all cases we see that the values of P are greater than the allowed values 0.05. These values are also presented graphically (Figure 9).
Figure 7: Graphic presentations of urine and Creatinine values in patients with diabetic nephropathy and patients in the con-trol group.
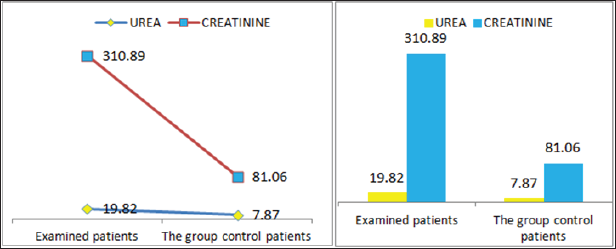
Figure 8: Graphic representations of total protein counts in albumin in patients with diabetic nephropathy and in control group patients.
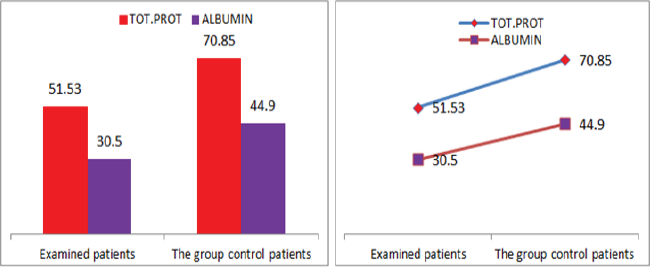
Figure 9: Graphic presentations of transaminase values in patients with diabetic nephropathy and patients in the control group.
Result 1: Paired T-Test and CI: 1 Initial Proteinuria gr/l, 2 Initial Proteinuria ; gr/l .
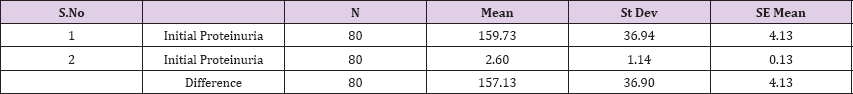
95% CI for mean difference: (148.92, 165.34)
T-Test of mean difference = 0 (vs # 0): T-Value = 38.09, P-Value = 0.000
Paired T-Test and CI: 1 Initial Proteinuria gr/l, 2 Initial Proteinuria; gr/l
Paired T for 1 Initial Proteinuria gr/l - 2 Initial Proteinuria; grl
Result 2: Paired T-Test and CI: 1 Proteinuria after 3 months, gr/l, 2 Proteinuria after 3 months
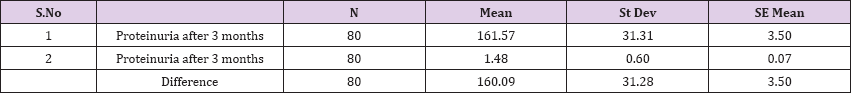
95% CI for mean difference: (153.13, 167.05)
T-Test of mean difference = 0 (vs # 0): T-Value = 45.78, P-Value = 0.000
Paired T-Test and CI: 1 Proteinuria after 3 months, gr/l, 2 Proteinuria after 3 months; g/l
Paired T for 1 Proteinuria after 3 months, gr/l - 2 Proteinuria after 3 months; g_1
Result 3: Paired T-Test and CI: 1 Initial Proteinuria, gr/l, 2 Proteinuria after 6 months, gr/l
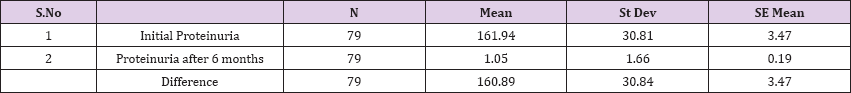
95% CI for mean difference: (153.98, 167.80)
T-Test of mean difference = 0 (vs # 0): T-Value = 46.37 P-Value = 0.000
Paired T-Test and CI: 1 Initial Proteinuria, gr/l, 2 Proteinuria after 6 months, gr/l
Paired T for 1 Initial Proteinuria, gr/l - 2 Proteinuria after 6 months, gr/l
Result 4: Paired T-Test and CI: 1 WBC, 2 WBC.
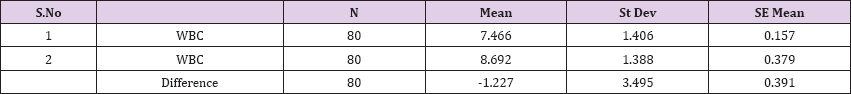
95% CI for mean difference: (-2.004, -0.449)
T-Test of mean difference = 0 (vs # 0): T-Value = -3.14, P-Value = 0.002
Paired T-Test and CI: 1 WBC, 2 WBC
Paired T for 1 WBC - 2 WBC
Result 5: Paired T-Test and CI: 1 RBC, 2 RBC.
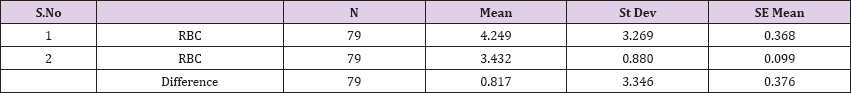
95% CI for mean difference: (0.067, 1.566)
T-Test of mean difference = 0 (vs # 0): T-Value = 2.17, P-Value = 0.033
Paired T-Test and CI: 1 RBC, 2 RBC
Paired T for 1 RBC - 2 RBC
Result 6: Paired T-Test and CI: 1 HGB, 2 HGB
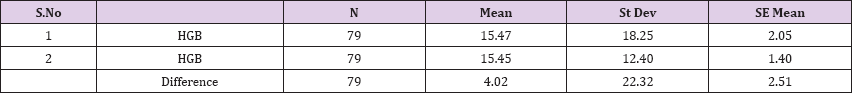
95% CI for mean difference: (-0.98, 9.02)
T-Test of mean difference = 0 (vs # 0): T-Value = 1.60, P-Value = 0.113
Paired T-Test and CI: 1 HGB, 2 HGB
Paired T for 1 HGB - 2 HGB
Result 7: Paired T-Test and CI: 1 HCT, 2 HCT.
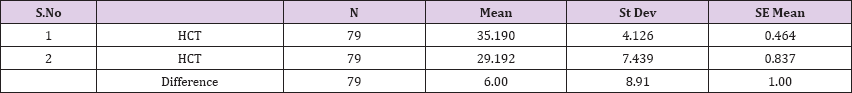
95% CI for mean difference: (4.00, 7.99)
T-Test of mean difference = 0 (vs # 0): T-Value = 5.98, P-Value = 0.000
Paired T-Test and CI: 1 HCT, 2 HCT
Paired T for 1 HCT - 2 HCT
Result 8: Paired T-Test and CI: 1 Creatinine, 2 Creatinine.
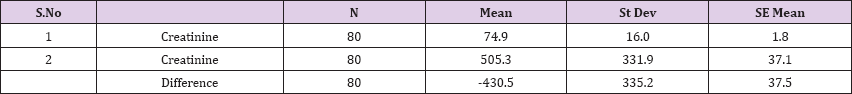
95% CI for mean difference: (-505.0, -355.9)
T-Test of mean difference = 0 (vs # 0): T-Value = -11.49, P-Value = 0.000
Paired T-Test and CI: 1 Creatinine, 2 Creatinine
Paired T for 1 Creatinine - 2 Creatinine
Result 9: Paired T-Test and CI: 1. Total Proteins, Total Proteins.
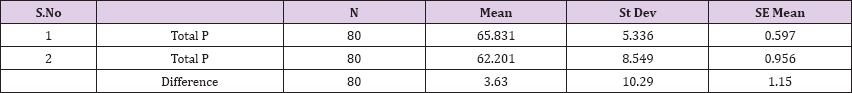
95% CI for mean difference: (1.34, 5.92)
T-Test of mean difference = 0 (vs # 0): T-Value = 3.15, P-Value = 0.002
Paired T-Test and CI: 1 Total Proteins, Total Proteins
Paired T for 1 Total P-Total P
Result 10: Paired T-Test and CI: 1 Uric Acid. 2 Uric Acid.
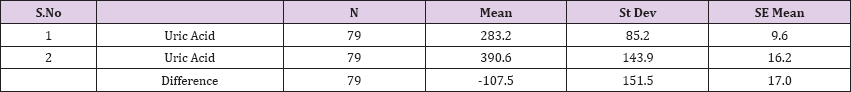
95% CI for mean difference: (-141.4, -73.6)
T-Test of mean difference = 0 (vs # 0): T-Value = -6.3, P-Value = 0.000
Paired T-Test and CI: 1 Uric Acid, 2 Uric Acid
Paired T for 1 Uric Acid - 2 Uric Acid
Result 11: Paired T-Test and CI: 1 ALT, 2 ALT.
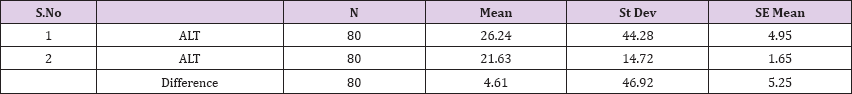
95% CI for mean difference: (-5.83, 15.05)
T-Test of mean difference = 0 (vs # 0): T-Value = 0.88, P-Value = 0.382
Paired T-Test and CI: 1 ALT, 2 ALT
Paired T for 1 ALT - 2 ALT
Result 12: Paired T-Test and CI: 1 AST, 2 AST.
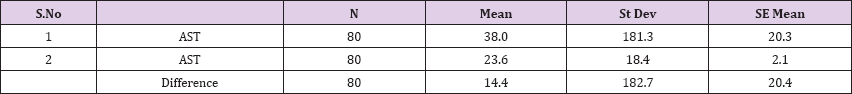
95% CI for mean difference: (-26.3, 55.1)
T-Test of mean difference = 0 (vs # 0): T-Value = 0.71, P-Value = 0.483
Paired T-Test and CI: 1 AST, 2 AST
Paired T for 1 AST - 2 AST
Result 13: Paired T-Test and CI: 1 Albumin, 2 Albumin.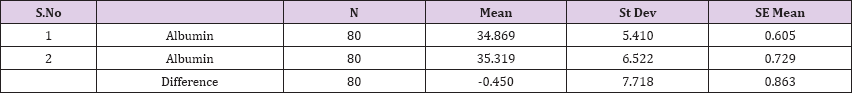
95% CI for mean difference: (-2.168, 1.268)
T-Test of mean difference = 0 (vs # 0): T-Value = -0.52, P-Value = 0.603
Paired T-Test and CI: 1 Albumin, 2 Albumin
Paired T for 1 Albumin - 2 Albumin
Result 14: Paired T-Test and CI: 1 Cholesterol, 2 Cholesterol.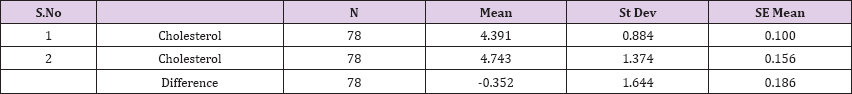
95% CI for mean difference: (-0.722, 0.019)
T-Test of mean difference = 0 (vs # 0): T-Value = -1.89, P-Value = 0.063
Paired T-Test and CI: 1Cholesterol, 2Cholesterol
T for 1 Cholesterol-2 Cholesterol
Result 15: Paired T-Test and CI: 1 Triglycerides 2 Triglycerides.
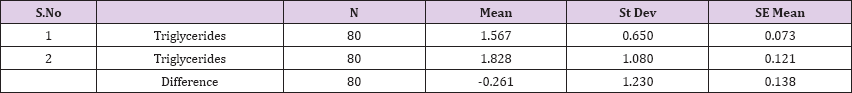
95% CI for mean difference: (-0.534, 0.013)
T-Test of mean difference = 0 (vs # 0): T-Value = -1.90, P-Value = 0.062
Paired T-Test and CI: 1 Triglycerides 2 Triglycerides
Paired T for 1 Triglycerides - 2 Triglycerides
All 162 patients with type 2 diabetes and renal insufficiency after treatment with Pentoxiphyllin 400 mg, three times a day have decreased proteinuria or reduced protein loss in the urine in a significant way. Although all these patients were treated with ACE or ARB, the possibility that the main factor of protein reduction was ACE or ARB, even in the second quarter, which means after the 6-month study we see that there is much more significant decrease of protein loss through urine compared with treatment initiation values, also with the value of the first quarter of treatment it is proved that the effect of Pentoxypilline in decreasing the loss of protein in the urine, it is confirmed by the healthy control group that expose the study.
Comparison of T-Test lab tests among patients treated with Pentoxiphylline 400 mg and patients with placebo statistical difference had leukocytes, erythrocytes, Hematocrit, Creatinine, total protein and uric acid, while the values of laboratory analyzes that did not differ (ALT, P-Value = 0.382), AST values (AST, P-Value = 0.483), Albumin values, P- Value = 0.603), Cholesterol Values (Cholesterol, P-Value = 0.063) and triglyceride values (Triglycerides, P-Value = 0.062). In all cases we see that the values of P are greater than the allowed values 0.05. Based on the results of the survey, we conclude that Pentoxiphylline 400 mg, three times a day therapy has a good effect on decreasing protinunuria and preventing kidney failure.
The applied dose is not toxic and has no kidney effects. The results of our study are sufficiently consistent with the results of many authors in their papers published in scientific journals such as ERA-EDTA or JAS, Nephrology etc.


