Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Franca Patrizi*1, Anna Maria Polifemo1, Cecilia Binda2, Shyam Dang3and Vincenzo Cennamo1
Received: February 20, 2018; Published: February 27, 2018;
*Corresponding author: Franca Patrizi, Colon Unit, AUSL Bologna, Italy
DOI: 10.26717/BJSTR.2018.02.000805
Colonoscopy is one of the most performed procedures in gastroenterology practice and is the criterion standard for colorectal cancer (CRC) prevention, used either for primary or secondary screening. The efficacy of colonoscopy and the preventive effect are highly dependent on the quality of the examination. Complete and accurate reporting of colonoscopy parameters is essential to assess the quality of the procedure and its improvements over time. Moreover, comprehensive reporting analyses can identify possible causes of shortfalls and direct specific training or education projects to achieve the maximum benefit from colonoscopic procedures [1-3].
Starting from the publication of guidelines on reporting practice in 2007 [4] many studies have focused the attention on quality evaluation and quality improvement of colonoscopy reporting, in screening programs or daily clinical practice [5-7]. Computerized reporting programs with mandatory text fields have been demonstrated to easily improve the quality of reporting [8,9]. Also, educational intervention was successful in improving reporting of lowest compliance quality indicators [10,11]. In our study we applied the PolaRis system, a new computerized system with compulsory items that obliged endoscopists to report an essential set of structured items, including key quality indicators. We aimed at demonstrating that forcing compliance to quality indicator reporting translates not only in higher quality in documentation practice but also in intervention on the endoscopist based on psychological and educational traits, resulting in a higher number of colonoscopies considered adequate for bowel cleaning.
Abbrevations: CRC: colorectal Cancer; ADR: Adenoma Detection Rate; ESGE: European Society of Gastrointestinal Endoscopy; PDR: Polyp detection rate; SPR: suboptimal preparation rates
The Colon Unit-AUSL Bologna, Italy is a tertiary care institution with a 4-room endoscopy staffed with 11 medical endoscopists. On average, in our unit we perform 5500 colonoscopy per year and we have been selected as an endoscopic center for regional colorectal cancer screening program since 2005. All the endoscopists who work in the unit are gastroenterologists with a minimum 7-years experience in endoscopy and an adenoma detection rate (ADR) of at least 20% in an average screening population (data from Emilia Romagna regional screening program).
Based upon quality indicators suggested by the European Society of Gastrointestinal Endoscopy (ESGE) to be included in a screening colonoscopy report, we developed a specific list of items (Table 1) to be explicitly addressed in the colonoscopy reports. From this list, PolaRis system, a new electronic reporting system, was developed in collaboration with EL.CO Srl (Cairo Montenotte, Italy). Since October 2013 our Unit is equipped with this structured computerized endoscopic report program which is the only one used for colonoscopy reports by all endoscopists.In the new reporting system, all defined quality indicators are mandated to be reported. Only few data items might be acquisited by free text entries (patient history, assessment of patient risk and comorbility, conclusions) while the essential items for quality reporting have to be recorded using preset terminology from drop-down menus. The final report is generated only after all fields have been selected. Since all items described carry specific codes, this enables regular production of standard analyses for quality assurance and benchmarking.
Table 1: PolaRis report: Colonoscopy Quality Indicators.
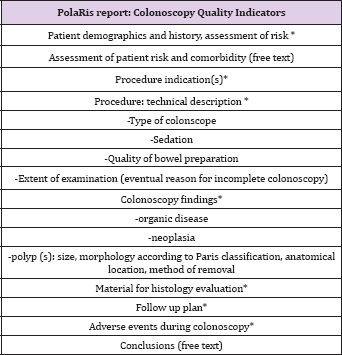
Bowel preparation was scored using the definitions "excellent," "good," "fair," and "poor," according to regional screening program guidelines [12]. Adverse events occurring at the time of colonoscopy have to be mentioned while the histolopathologic diagnosis is not included since, so far, there is not an automatic link between pathology files and our endoscopy reporting system. Follow up plan has to be registered on the final report with fixed terminology, but, in case of awaited histological examination, it is not conclusive. Table 2: Patient and Colonoscopy Characteristics.For polyp dimension, there is the possibility either to report the diameter in millimetres or to select the item "subcentimetric" (if the diameter is inferior to 10 mm) since, according to regional guidelines this is the dimensional information needed to define follow up. Paris classification is used to describe polyp morphology [13] . For polypectomies, it is necessary to select the method (forceps, snare, endoscopic mucosal resection or endoscopic submucosal dissection) for polyp removal and if the procedure was performed en bloc or piecemeal.In case of screening program patients, there is a different reported feedback either for adverse events occurring in one month time after the procedure or for histopathology reports/ follow up plans after evaluation of histopathology. However, these data cannot be added in the colonoscopy report once it has been validated with digital signature.
We designed an observational retrospective study consisting of two phases. In the first phase, we conducted a chart review of all consecutive colonoscopy reports prior to the introduction of computerized PolaRis reporting system, from September 30, 2013 backward until we reached 600 colonoscopies (300 in FOBT-based screening program patients and 300 in non-screening inpatients or outpatients), spanning back to July 20, 2013. Considering a two month period of training for optimal use of the system, 600 consecutive colonoscopy reports (300 FOBT-based screening and 300 non-screening patients), from December 1, 2013 to January 28, 2014, were retrospectively informatically reviewed, with the assistance of EL.CO. Srl. In the study we only excluded reports on therapeutic colonoscopies performed after diagnostic exams.
Testing was performed using the Student s t test for continuous variables and the x2 test for categorical variables. Data are presented as means and SDs for continuous variables and proportion and 95% CIs for categorical variables. Quality of bowel preparation was considered as "adequate" when preparation were defined as "excellent" or "good", as "inadequate" if described as "fair" or "poor",since according to regional guidelines in case of fair or poor preparation the procedure has to be repeated. Statistical analyses were performed using SPSS for Windows release 10.0 (SPSS INC., Chicago, IL).
Table 2: Patient and Colonoscopy Characteristics
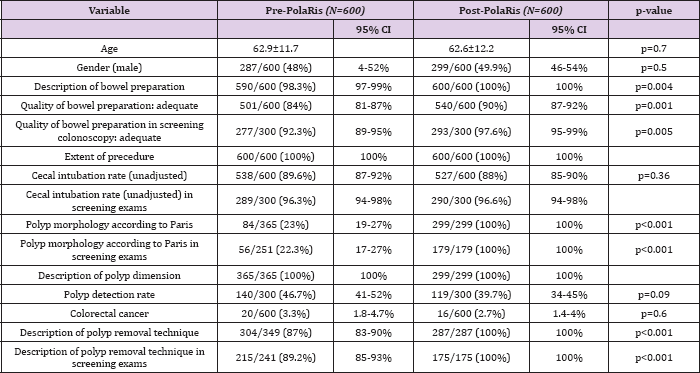
From July 13, 2013, 600 consecutive colonoscopy reports about 300 patients from screening program and about 300 nonscreening patients were analyzed group 1. After introduction of the new reporting system, from December 1, 2013, 600 colonoscopy reports about 300 patients from screening program and about 300 non-screening patients were evaluated group 2. These reports were filled by 11 different experienced gastroenterologists. A summary of patient and colonoscopy characteristics is depicted in Table 2. The distribution of age (p=0.7) and gender (p=0.5) were similar between pre- and post-PolaRis intervention groups. The quality of the bowel preparation was not recorded in 10/600 (3 screening patients and 7 non-screening patients) of the reports in group 1. In group 2, 100% of the reports included this record (p=0.004). In group 1 the quality was described as adequate in 501/600 exams (92.3% in screening pts and 74.6% in non-screening patients, total 84%). In 57 procedures in group 1 (9.5%) (11 screening and 46 non screening) the definition of the quality of preparation was "personalized" by the endoscopist with descriptive phrases (that can easily be interpreted as "inadequate" preparation: i.e: "preparation not complete in many areas", "preparation not achieving acceptable level") instead of using the established grading system. In group 2, bowel preparation was indicated as adequate in 540/600 cases (97.6% in screening pts and 82.3% in non-screening patients, total 90%), the field was obliged so all preparations were described according to defined grading system. The difference in the two groups regarding the adequacy of bowel preparation was statistically significant (p=0.001).
Considering only screening procedures, colonoscopy with 'fair' preparation were 20/300 in group 1 and 5/300 (p=0.004) in group 2. Procedures with 'good' preparation were improved from 142/300 in group 1 to 167/300 in group 2 (p=0.0049). 'Excellent' (135/300 vs 126/300, p>0.36)) and 'poor' (0/300 vs 2/300) subgroups in screening population did not differ statistically before and after computerized system introduction (Table 3). All reports in group 1 and 2 included information regarding cecal intubation which was not statistically different between the two groups. In group 2, the cecum was reached in 88% of colonoscopies (96.6% in screening subgroup and 79% in non-screening subgroup); in group 1, 89% of colonoscopies were completed to the cecum (96.3% in screening subgroup and 83% in non-screening subgroup) (completely unadjusted data as recommended in ESGE guidelines) [14] . Polyp detection rate (PDR) in screening procedures was 46.7 % in group 1 and 39.7% in group 2 (140/300 vs 119/300, p=0.09, not statistically different).
PDR in screening procedures was not significantly different in the adequate and inadequate preparation subgroups before and after PolaRis use (134/277 adequate colonoscopies with polyps /adequate colonoscopies in group 1 and 119/293 adequate colonoscopies with polyps/adequate colonoscopies in group 2, p=0.07).Polyp dimension was always described in group 1 (365/365) and in group 2 (299/299), expressed in millimetres or with the term "subcentimetric" if diameter was inferior to 10 mm. Paris classification for description of polyp morphology was applied in 23% of polyps detected in group 1 (84/365) and in 100% of polyps detected in group 2 (299/299, p<0.001). The method of polyp removal was not mentioned in 45/349 reports with polypectomy (12.9%) in group 1 while in group 2, the technique of removal was indicated in all 287 cases of polypectomy (100%, p<0.001). Detection of colonic cancer (excluded T1 cancers detected at histology) was comparable in the two groups (p=0.6) (Group 1 in 20/600 cases and group 2 in 16/600 cases).
Table 3: Bowel Preparation Description in Screening Colonoscopy.

Our study shows that the use of a structured system for colonoscopy reporting directly improves both the colon cleansing reporting rate and the adequate colon preparation rate. Following the introduction of the PolaRis system, not only the quality of preparation was mandatory described in every report (100% of reports), it was always assessed in a standardized way (100% of reports) and, much interestingly, this quality was also considered adequate in a much higher number of reports than with free text reporting (90% vs 84%, p=0.001). The description of the quality of bowel preparation is a required element of the colonoscopy report [4,15]. Indeed, an incomplete documentation of the adequacy of bowel preparation may lead to repeat examination after a shorter interval than that advised by the guidelines, resulting in extra discomfort for patients and in economic disadvantage for the health system. To improve the quality of endoscopic reporting, scientific societies developed guidelines that described a specific list of quality indicators to be explicitly addressed in the colonoscopy reports [14,16]. Nevertheless, to date, reporting on the quality of colonoscopy in daily clinical practice has been disappointing. Important items such as the quality of bowel preparation, documentation of cecal landmarks, polyp features and the number of polyps found are often lacking [9,17,18]. In the study of de Jonge et al only 62% of the reports mentioned this item (range between departments 7%-100%) [3]. Even when correctly reported, bowel cleansing is inadequate in up to 20-25% of all colonoscopies [19].
It is well known that the evaluation of bowel cleansing is strongly operator-dependent [20]. Indeed, Ben-Horin et al. [21] demonstrated significant interobserver variability on classification of same bowel preparation, showing that the assessment of colon cleanliness varies considerably among endoscopists observing identical colonscopic images. Published bowel preparation scales rely on factors prone to inter-observer variation such as quantitative estimates of residual stool or liquid, the percentage of visualized mucosa, or the likelihood of missing certain sized lesions [22]. In clinical practice, providers often use an imprecisely defined 4-point scale of excellent, good, fair and poor. In this scheme, preparations described as excellent and good are widely viewed as adequate but some research indicates that many fair preparations are also adequate [23]. Mahadev et al in 2014 [24] found that rates of suboptimal (rated poor/unsatisfactory or fair on reports) preparation in screening colonoscopy vary widely among providers, ranging from 3% to 40%. For this reason strategies to improve performance and correctly define the bowel cleansing have been suggested [10]. ASGE /ACG taskforce recommends that the examination be considered adequate if it allows detection (within the technical limitations of the procedure) of polyps >5 mm, after suctioning and washing the mucosa [17].
To date, our study is the first that shows that structured reported system could improve the adequate prepartion rate in colonoscopy. Our interpretation of adequate preparation rate improvement is linked to the "Hawthorne effect", the psychological phenomenon in which the awareness of being observed may alter the practice of individuals. In medicine too, it is well known that the simple act of monitoring a service, improves performance in a powerful and essentially free way [25]. Translating this effect to our study, when endoscopists have to complete automated colonoscopy reports with mandatory preparation-quality grading system and mandatory follow-up plans, and are aware of the possible quality audit, they eventually modify their behaviour (maybe through washing and aspiration of fecal residuals) and their final perception of preparation adequacy.
The structured system implementation seems to increase colon cleanliness without increasing PDR. Indeed, the standardized reporting system does not appear to have additional benefits on PDR for screening colonoscopy (46.7% vs 39.7% p=0.09, not statistically different). In our study, we could not calculate the adenoma detection rate because of the lack of histopathological feedback. Anyway, PDR correlates well with ADR in several studies with the advantage of not requiring manual entry of pathological data [17]. Clark et al. [26] performed a systematic review and meta-analysis to assess the adequacy of a fair-quality bowel preparation finding no differences in the ADR of colonoscopies with an intermediate (fair) quality bowel preparation compared to those with a high quality (excellent/good) preparation, suggesting the need for early repeat colonoscopy only with low-quality (poor/insufficient) bowel preparation. Mahadev et al. [24] found that rates of suboptimal preparation (rated poor/unsatisfactory or fair on reports) not only vary widely between providers but also did not correlate with ADR. This suggested that a high standard for grading preparation as optimal, and hence higher suboptimal preparation rates (SPR) is not a marker of higher quality standards and expectations by the provider. The authors concluded that undefinied factors -including personal and professional traits -likely contribute to both ADR and SPR require further study. We interpret previous and actual results, analyzing the subgroups of reporting in screening procedures for what concerns PDR and adequate/inadequate bowel preparation description. The difference between group 1 and 2 regarding bowel preparation was mainly among "good" and "fair" preparation subgroups. Colonoscopy with fair preparation were reduced from 20/300 to 5/300 (p=0.004) and those with good preparation were improved from 142/300 to 167/300 (p=0.005). Interestingly, if we relate PDR in screening procedures to the adequacy of bowel preparation, we find that PDR was not significantly different in the adequate and inadequate preparation subgroups (134/277 adequate colonoscopies with polyps/adequate colonoscopies in group 1 and 119/293 adequate colonoscopies with polyps / adequate colonoscopies in group 2, p=0.07).
We believe that both the mandatory description of indicators (i.e. quality of colon preparation and follow up suggestions) and the awareness of being assessed through the computerized system,strenghten the link between appearance and perception of the provider, with a psychological effect on him to conclude for higher number of adequate bowel preparation, forcing the shift of an amount of 'fair' preparations into the 'good' subgroup. This does not affect quality as measured by PDR (as for ADR in previous studies). Apart from bowel preparation, our study shows that the use of PolaRis system for colonoscopy reporting also directly improves the rate of other reported quality indicators. Meaningfully, the use of Paris classification for polyp morphology prior to the introduction of PolaRis system were very limited (23% vs 100%, p<0,001), in screening procedures too. Also, the description of polypectomies was significantly less available in the free text reporting (group 1 total 87% vs group 2 total 100%, p<0.001), being more often reported in screening (89.20%) versus non screening (82.4%) procedures.
This information impacts the choice of surveillance intervals and becomes a critical driver of appropriate resource utilization in population-based screening program [15]. Moreover, accurate description of the endoscopic appearance of polyps by using a standardized classification system (Paris classification), the definition of size for complex colon polyps and its method of removal (ie: en bloc or piecemeal) [27] may guide providers in the decision between curative endoscopic resection versus need for surgery [28]. The high compliance in other quality indicators (cecum intubation rate, polyp dimension) in prePolaRis era was higher compared to other studies [3]. This is surely related to our long experience in colorectal cancer screening (we are a selected unit for regional colorectal cancer screening program since 2005) with special attention in addressing all the basic information needed to establish follow-up for screening patients., This attitude reflected as well on non-screening procedures where the compliance in these indicators reporting was satisfactory too. Regarding comparable performance parameters as the cecal intubation rate, it was not significantly modified by the use of standardized reporting. Reasonably, computerized reporting obliged this quality items to be described, becoming available for quality assurance, but it does not influence the indicator rate since this is a performance indicator and is not susceptible to subjectivity.
We could not compare and measure other reporting or performance indicators (pre-procedural items, indication for exams, etc) before and after the introduction of new computerized reporting program since only rarely (data not shown) these were described before October 2013 on free text reports. In conclusion, to our knowledge this study, for the first time shows that a structured, mandatory drop-down menu reporting system for colonoscopy improves not only the rate of reported quality indicators but also the accuracy of procedures, influencing the adequate colon preparation rate. Further studies are needed to assess the influence of these results on clinical outcome, follow-up guidelines adherence and cost-effectiveness of colonoscopy.
One limitation ofthis study is that, assuming that all endoscopists were experts in colonoscopy, we focused on quality reporting and performance per unit and did not report on endoscopist- specific performance quality. Future quality audits, simplified by computerized analyses of reports, could make more evidence available on the quality of reporting and quality of performance of endoscopists individually. Among the main limitations of Polaris software, there is the lack of histopathological diagnosis on the endoscopic report. Through data analyses we could not differentiate among adenomatous or non adenomatous polyps, neither we could evaluate T1 adenocarcinoma found at histology. We are planning in the future a link with pathology reports to create a final version of the colonoscopy report updated with the histological analyses and follow-up details based on endoscopic and microscopic findings.
(Tables 1-3)


