Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Omar Rashid Sadeq*
Received: January 28, 2018; Published: February 12, 2018
Corresponding author: Omar Rashid Sadeq, Assistant Professor, Faculty of Dentistry, Arab American University Jenin, (AAUJ) Palestine
DOI: 10.26717/BJSTR.2018.02.000752
Piroxiam is an oxicam derivative medication belonging to non steroidal anti-inflammatory drugs (NSAIDs) group, used to treat moderate to severe inflammatory diseases such as rheumatoid arthritis, osteoarthritis, ankylosing spondylitis (Bechterew's disease), tendinitis, bursitis, and for pain that is not related to musculoskeletal system e.g. primary dysmenorrhea and postoperative pain. It reduces pain, joint swelling, morning stiffness, and improves the functionality of the joints during chronic polyarthritis. Piroxicam has been compared to other antiinflammatory agents (e.g. diclofenac, indomethacin, and naproxen) in numerous controlled studies and proved to be equal and sometimes even more efficacious. Piroxicam is unique among NSAIDs in that it is used once daily, and its efficacy is equal to most important clinically employed NSADs (ibuprofen, naproxen and diclofenac) especially in amelioration of postoperative pain, but it is more prone to causing gastrointestinal disturbances and serious skin reactions (Steven-Johnsons syndrome, toxic epidermal necrolysis). It should not be given to patients who have experienced peptic ulcer, asthma, urticaria or allergic-type reactions after taking aspirin or other NSAIDs, as well as patients on anticoagulant therapy, renal, hepatic, diabetic and cardiac patients.
Piroxicam is not recommended for use in lactation, children under 12 years of age and pregnant women since safety has not been established as with any NSAID, caution should be exercised in treating the elderly (65 years and older). Piroxicam is provided in much trade formulation namely feldene, Felcol and pirox, it is available in multiple dose variations 10-20 mg capsules PO 1-2 times/day (no more than 30-40 mg/day) and is taking with or after food, the dose schedule is individual and depends on pathologic condition. Clinically significant drug interactions with piroxicam include bleeding when used with either anticoagulants or other NSAIDs, decreased efficacy of antihypertensive (ACEIs and beta blockers but not Ca channel blockers), reduced the antinatriuretic effect of diuretics such furasemide and hypothiazide, acceleration of the steady state of digoxin, increased nephrotoxicity of methotrexate and cyclosporine and finally concomitant use of corticosteroids with Feldene may increase the risk of GI ulceration or bleeding. The aim of recent study was to estimate the hepatic risk associated with the use of piroxicam, about 32 patients with osteoarthritis (OA) were divided into 2 categories, and were treated by piroxicam, 10-20 mg/d orally in a period of 2 months, patients were investigated for 6 month. CBC, hepatic tests including (bilirubin, ALT and AST) were done before, during and after treatment, ultrasonography and liver biopsy was also provided only for certain patients of II category. The main findings of this research study is that two-month orally prescribed piroxicam, 10 mg/d produces no hepatotoxic effect in OA, but piroxicam, 20 mg/d, produces a mixed hepatocellular-cholestatic reversible injury in about 75% of OA patients, predominantly in female gender.
Keywords: Piroxicam; NSAIDs; Osteoarthritis; Rheumatoid Arthritis; Dysmenorrhea; Polyarthritis; Steven-Johnsons syndrome; Furasemide Hypothiazide; Methotrexate; Digoxin; ultrasonography; cholestasis; Liver Biopsy
Abbreviations: DILI: Drug Induced Liver Injury; NSAIDs: Non-steroidal anti-inflammatory drugs, CIX: Cyclooxygease; ALT: Alanine Amino Transferase; CBC: Complete Blood Count; RF: Rheumatoid Factor; ALP: Alkaline Phosphatase
The liver plays an astonishing array of vital functions in the maintenance, performance and regulating homeostasis of the body, its major functions are immunity, carbohydrate, protein and fat metabolism, exogenous (drug) and endogenous substances detoxification, secretion of bile and storage of vitamin. More than 900 drugs have been implicated in causing liver injury and it is the most common reason for a drug to be withdrawn from the market. Drug Induced Liver Injury (DILI) is progressively increased, general pathophysiologic mechanisms involved in DILI include [1-5],
a) Direct injury of hepatocytes with their membrane rupture
b) Interruption of bile flow via blocking of transport proteins at the canalicular membrane
c) Apoptosis of hepatocytes
d) Immunologic when a drug act an immunogen, and can affect the P450 system
e) Bile duct injury, the most commonly DILI are antibiotics,
NSAIDs, anesthetic agents, antihyperlipedmics, antirheumatic drugs, TNF inhibitors, antiepileptics, antpsychotic drugs acetyl cholinesterase inhibitors, tricyclic antidepressants and antihypertensive agents [6-10].
Non-steroidal anti-inflammatory drugs (NSAIDs) are consumed massively worldwide and, along with antimicrobial agents, are the most frequent causes of drug-induced liver injury. The pharmacology of NSADs is broad and diverse, their principle effects are analgesic, antipyretic and anti-inflammatory, these effects are highly advantages in the clinical settings, so many patients are afflicted by one or more of these symptoms. For this reason NSAIDs find use in conditions such as toothache, arthralgia, myalgia, headache, migraine, fever, to inflammatory arthritic diseases such as rheumatoid arthritis, osteoarthritis, gout, to postoperative pain.
More than 20 different NSAIDs are available commercially, and these agents are used worldwide, for their three above mentioned therapeutic effects in patients with multiple medical conditions. The prototype is aspirin, commonly prescribed NSAIDs are diclofenac K or Na, ibuprofen, indomethacin, piroxicam, naproxen, meloxicam and etoricoxib, and they share a common mode of action which involves blocking cyclooxygease (COX) enzymes [1116]. Different NSAIDs inhibit COX isoenzymes COX-1, COX-2 to different extents and this differential mechanism of action explains their differing wanted therapeutic effects and unwanted adverse actions. Acetaminophen (paracetamol) is not considered as NSAIDs as it has negligible anti-inflammatory powers even at high doses, (compared to aspirin), its analgesic and antipyretic effects are due to central inhibition of COX-1.
COX-1 isoenzyme is a constrictively expressed, because it is involved in many physiological processes for instance GIT mucosa protection, platelets aggregation and potency of blood vessels. COX-2 in contrast to COX-1 is facultatively expressed mainly during inflammatory states, but this does not exclude its physiologic role in CNS, macula densa of renal tissues as well as ovaries and uterus. Most NSAIDs competitively inhibit both isoenzymes to some degree, though aspirin- as an exception irreversibly blocks its target. COX inhibition is vital, as its COX enzymes are responsible for generation of prostanoids-substances which include prostaglandins "PGs" (implicated in inflammation and anaphylaxis), prostocyclines (active in resolution phase of inflammation) and thromboxanes (mediators of vasoconstriction). Nonselective COX-1/COX-2 inhibitors e.g. aspirin, piroxicam and naproxen- target COX-1 and as a result gastric PGs levels are reduced and for this reason GIT symptoms are considerably more common, ranging from mild erosions to severe bleeding, about 15 % of patients experience dyspepsia on NSAIDs, the use of misoprostol (PG analogue) with naproxen, diclofenac or aspirin protects GIT mucosa from ulcerogenic effects of NSAID [17-22].
Their antipyretic effect is due to inhibition of PGE-2 synthesis form the thermoregulatory center- the hypothalamus, but not hyperthermia in which the set point is not altered. Selective COX-2 inhibitors such as celecoxib, valdecoxib and meloxicam in low doses are superior to nonselective in that they have less GIT distress, without affecting the bleeding time and therefore preferred for patients suffering from GIT and bleeding disorders, on the other hand selective COX-2 inhibitors shouldn't be used in CNS and renal diseases.
They kill pain peripherally due to their ability to minimize sensitization of receptors to bradykinin and PGs, but not centrally as do opioids that interrupt pain transmission at the level of CNS, NSAIDs (non opioid narcotics) differ from opioids e.g. (morphine or fentanyl) in that they do not produce a ceiling effect, as well as NSAIDs don't affect the emotional aspect of pain and. COX inhibitors as monotherpay are ineffective in reducing visceral, spastic, ischemic, necrotic and neoplastic pains. Sometimes they are combined with opioids to reduce pain arising from non- integumental structures, and finally addiction and tolerance are specific for opioids rather than NSADs [23-30].
Pharmacokinetic ally, NSAIDs are well absorbed from the gastrointestinal tract, with the exception of aspirin (and possibly diclofenac) which undergoes presystemic hydrolysis to form salicylic acid. Concomitant administration of NSAIDs with food or antacids may in some cases lead to delayed or even reduced absorption, they are highly bound to plasma proteins (mainly albumin), which limits their body distribution to the extracellular spaces, undergo hepatic transformations variously by CYP2C8, 2C9, 2C19 and/or glucuronidation. Half-lives of the NSAIDs vary but in general can be divided into "short-acting” (less than 6 hours, including ibuprofen, diclofenac and indomethacin) and "long-acting" (more than six hours, including naproxen, celecoxib, meloxicam and piroxicam).The elimination of these drugs depends largely on hepatic biotransformation; renal excretion of unchanged drugs is usually small (less than 5% of the dose). NSAIDs differ in potency, duration of action, side-effect profile, and potential for drug interactions, the selection of NSAID should be based on clinical experience, patient convenience (e.g. once or twice daily dosage schedule), side effects and cost. Despite the increasing number of NSAIDs available, there are few data comparing the old and new agents for efficacy and safety, and there are few guidelines governing choices of NSAIDs for particular patients [31-37].
Many studies show that aspirin, indomethacin, naproxen, fenoprofen, and ibuprofen were equally effective in the treatment of RA. Other studies for management of osteoarthritis reveal that indomethacin, naproxen, isoxicam (chemical analogue of piroxicam) and ketoprofen are equal in efficacy but the latter three had fewer side effects than indomethacin. Naproxen and aspirin are preferred for the treatment of muscle contraction headache, whereas indomethacin should be avoided, in contrast, indomethacin is the drug of choice for chronic paroxysmal hemicrania and hemicrania continua. Studies show that piroxicam (20mg /day) compared to other NSAIDs is more potent and less frequently employed daily, because of its long half life, notably piroxicam in RA is equal to ibuprofen (400 mg 3-4 times a day), but better than indomethacin (25 mg administered three times daily). In OA, piroxicam is slightly superior to naproxen (500 mg B.I.D). There is a high degree of "crosssensitivity” between aspirin and other NSAIDs in patients who have symptoms of rhinitis or asthma, the genesis is pharmacologic rather than immunologic, compared to urticaria (upon exposure to aspirin) in which mechanism is probably is immunologic (salicylate metabolite), that does not correlate with other NSAIDs [38-43].
There is no proved advantage to using more than one NSAID at a time unless a rapid onset of action is needed. If one drug does not prove efficacious after 1-3 weeks at the maximally tolerated dose, another agent should be substituted. For patients with gastric intolerance to one NSAID, alternative therapy from another class should be considered. If unsuccessful, therapy with choline salicylate, salsalate or enteric-coated aspirin may prove useful. When adverse effects of NSAIDs on platelets are of concern, sulindac or ibuprofen should be considered, with nonacetylated salicylates as alternatives. If renal function is compromised, avoid NSAIDs, especially fenoprofen if possible; sulindac is perhaps the least offensive agent, but close monitoring should be instituted. When central nervous system side effects such as headache occur, aspirin or naproxen may be used. In hypertension the pressor effect of NSAIDs could be minimized by prescribing sulindac and avoiding indomethacin. Paracetamol is still the only analgesic choice for asthmatic patients. So the choice of any member of NSAIDs should be done carefully, assuming to the above mentioned factors.
About 32 primary knee joint OA patients aged from 30 to 60 years, were investigated for possibility of piroxiam-induced hepatitis in the period of 6 months, 16 of them are males, according to OA stage and symptoms piroxicam,10mg- 20mg/d was given for 2 months, because its therapeutic effects become evident, only after the first 8-12 weeks of treatment, the patients were categorized into 2 categories: The first category includes 16 patients with the (mild II stage) of OA, 9 of them are women, medicated orally by piroxicam,10 mg/day. The second category contains 16 patients with the (III stage moderate) of OA, 10 ofthem are women; they were given piroxicam 20mg/d. per.os. Complete blood count (CBC), the rheumatoid factor, (RF), hepatic tests functions including bilirubin, alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were done before during and upon completion of treatment in all patients. All patients were investigated for possibility of liver injury effect of piroxicam at the first 3-4 weeks following treatment. At the end of treatment, iron, ferttin, ultrasonography (US), serologic reactions and liver biopsy were indicated only to 11 jaundiced patients of the second category [44-47].
The first category of patients experience minor knee pain after walking and running, joint stiffness and tenderness, they were medicated by proxicam, 10 mg/ d for 2 months, the usual dose of piroxicam for OA arthritis is 20mg/d, but many studies show that giving piroxicam, 10 mg/d was successful in a small number of arthritic patients, the other purpose why we gave these patients this dose was to determine if piroxicam is dose related toxicant or not. Patients of the second category clinically complain of more intense symptoms compared to the first one, they manifest acute knee joint pain with swelling, the pain is exacerbated by motion and relieved by rest, night pain is said to be present in almost patients especially patients above 55 y, (8 females, 3 males), joint pain is typically accompanied by morning stiffness and generally lasts less than an hour, they also experience a decreased range of motion and muscle spam.
On examination, patients of II category demonstrate localized tenderness along the joint, osteophytes is palpable around the affected knee joint, before treatment, by piroxicam, all patients of I and II groups were investigated for possibility of the presence of cardiovascular, endocrine, hepatic, skin and renal diseases, all of which were excluded, a detailed medical history including thorough questioning about medical factors, risk factors, use of prescription drugs, self-medication, and use of unconventional substances such as alternative and herbal medicine was provided with negative answers. RF was negative in both categories. Investigations show that treatment by piroxicam in both categories, equally and markedly decrease signs of acute inflammatory process of knee joint. Patients of I group after treatment demonstrate improvement of motility, ability to flex or extend their knees, as well as decreased tenderness and joint stiffness. Along a period of two month treatment, only 2 of 16 patients demonstrate mild dyspepsia, abdominal pain, reflecting the ulcerogenic effect of piroxicam on GIT mucosa, any change in the skin or eye pigmentations are not noted in all patients of this category [48,49].
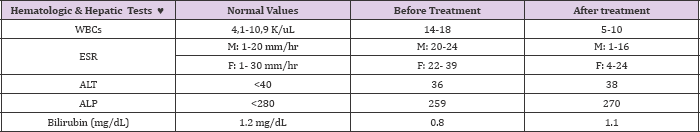
Piroxicam given to this group for 2 month at a dose 10 mg/d markedly decreases inflammatory reactions of OA, that was reflected on CBC, in which leukocytosis and ESR are normalized, indicating the efficacy of piroxicam, against OA, without any side effect on GIT mucosa, (except only in 12,5 % of patients dyspepsia appears, as mentioned above), because hepatic tests (bilirubin, and hepatic tests before , during and after treatment have no any significant deviation, therefore piroxicam, 10 mg/d for 2 month produces a strong effect against OA has no hepatotoxic effect (Table 1). Concerning the second category, piroxicam, 20 mg/d exerts a strong analgesic and anti-inflammatory effects in all patients, confirmed on CBC, by decreasing WBCs count as well as normalization of ESR, most patients demonstrate that motion becomes more better than before treatment, swelling, morning stiffness, night pain and muscle spasm are significantly diminished. Only 4 patients of this group demonstrate nausea and vomiting, other complaints are not observed, their hematologic analysis and biochemistry show no any significant deviation, before, during and after treatment. About 12 patients of this category (9 females, 3 males ) at the end of eight week of course treatment complained abdominal tenderness, fatigue, nausea, vomiting, generalized pruritus, jaundice, dark urine and pale stool, At this period, piroxicam therapy was stopped.
Table 1: Hematologic and Hepatic Tests of Patients of I Group Medicated by Piroxicam.
N.B
K/uL= one ul is equal to mm (3) K means a thousand (1000 cells/ul).
♥ : the symbol refers to mean average of laboratory values among 16 patients.
ALT: Alanine aminotransferase, ALP: Alkaline phosphatase, M: Male, F: Female.
The mean average of biochemical analysis was taken from the 12 jaundiced patients of the II category showed, increased total concentration of bilirubin, (5.9mg/dl), increased serum of bilirubin accounts for conjugated form, ALT and ALP levels are also increased (694, 575 U/L respectively), with normal CBC. Ultrasonography (ultrasound) showed a normal liver, gallbladder and biliary tree. Liver biopsy reveals intrahepatic cholestasis with only mild inflammation and hepatocellular necrosis (Table 2). Patients presented by acute symptoms of suspected hepatic injury at the end of the 8th week of treatment by piroxicam, had no history about hepatic diseases, they didn't take any medication for the last six month, serologic reactions to hepatitis A, B, C, cytomegalovirus, Herpes Simplex, Epstein-Barr virus were negative, values of iron and ferrtin are within the reference range, so excluding any infectious or metabolic disorder that could be a cause of hepatic injury.
Table 2: Laboratory Values of 12 jaundiced Patients of the Second

N.B
♥ : The symbol refers to mean average of laboratory values among 12 jaundiced patients of II category.
Alanine aminotransferase (ALT), alkaline phosphatase (ALP), Piroxicam 'P'
At the onset of symptoms, hepatic tests showed, elevated total amount of bilirubin, conjugated hyperbilirubinemia predominates unconjugated variant, normally, the total bilirubin level is less than 1.2 mg/dL (the reference range of direct bilirubin is 0.1-0.4 mg/ dL) and approximately 70% is indirect (unconjugated) bilirubin. Conjugated hyperbilirubinemia (>50% of the total bilirubin is direct) suggests hepatocellular dysfunction or cholestasis, Unconjugated hyperbilirubinemia (>80% of the total bilirubin is indirect) suggests hemolysis or Gilbert syndrome, when the bilirubin level is above 25-30 mg/d, extrahepatic cholestasis is an unlikely diagnosis; because the predominantly conjugated bilirubin is water soluble, it is easily excreted by the kidney in extrahepatic cholestasis. Levels of bilirubin in these patients begin gradually to be decreased after discontinuation of piroxicam therapy and normalization had occurred at the end of 4th month (Table 2).
Regarding ALT and ALP levels, at the onset of symptoms, ALT serum is increased more than ALP, a week later serum ALT is decreased, but the serum of ALP is increased, then at the end of the II week, levels of ALP are significantly elevated, compared to slightly increased ALT levels, and finally, the 20th day of prioxicam withdrawal therapy reveals that levels of both enzymes begin gradually to be decreased and by the end of 4th month are within normal limits in all patients, thus the R value which is employed to determine the relationship between ALT and ALP is 3.5, at the onset of symptoms, indicating a mixed hepatocellular-cholestatic pattern of injury, further elevations in ALP and a rapid decrease in ALT (by the 7th day) yielding R values of <2), generally when ALP is greater than twice the normal upper limit and R < 2, the type of injury is the cholestatic pattern, in hepatocellular pattern ALT is greater than twice the normal upper limit or R > 5, and 2 a) Drugs that directly affect the liver, and usually is dose related, e.g., acetaminophen. b) Idiosyncratic drug reactions: medications that promote hypersensitivity (immune) reactions due to either parent drug or its metabolite, the mechanism of piroxicam induced liver injury is not well known, but may be due to a toxic metabolic intermediate of piroxicam metabolism, which occurs largely in the liver. a) Nocturnal acute pain of OA present in most (75%) patients (above 55 years) of second group, and mainly dominant in women. b) Piroxiacam, 10-20 mg/d for 2 month duration therapy is a good medicament for treatment of OA (II and III stage), with an ulcerogenic effect (12,5%- 25%) in the first and second categories respectively. c) Piroxicam, 20mg/d decreases the incidence of nocturnal pain and may be muscle spasticity, which suggests it as one of most effective drugs against pain at night. d) Piroxicam, 20mg/d is throughout to cause hepatic injury at the end of 8th week of treatment in about 75% of patients belonging to the second category. e) Piroxicam, 20 mg/d causes a mixed hepatocellular- cholestatic pattern of injury, based on R values of ALT and ALP enzymes. f) The hepatic injury induced by piroxicam is reversible as jaundiced patients completely recovered and laboratory values returned to their normal limits, by the end of 4th month of piroxicam withdrawal therapy. g) The mechanism of piroxicam-induced hepatotoxicity is unknown, but strongly believed to be idiosyncratic character, rather than dose related mechanism, piroxicam, 10 ma/d causes no hepatic injury. h) It is recommended to control CBC indices as well as hepatic enzymes in patients on piroxicam therapy in the first 3-12 weeks in order to minimize its possible hepatotoxic effect.
Conclusion
References


