Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Edmond Nzene Mesumbe1*, Philip Njotang Nana1,2, Julienne Stéphanie Nouetchognou3, Julius Dohbit4, Evelyn Mah4, Flibert Eko Eko2, Jeanne Fouedjo2 and Agnes Essiene2
Received: January 25, 2018; Published: February 07, 2018
Corresponding author: Edmond Nzene Mesumbe, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Yaoundé, Cameroon, Africa
DOI: 10.26717/BJSTR.2018.02.000736
Background: Meconium stained amniotic fluid (MSAF) is frequent in obstetric and neonatal practice. It is responsible for maternal, foetal and neonatal complications. The objective of this study was to determine the association of MSAF with perinatal outcome.
Methods: This was a prospective cohort study targeting pregnant women admitted in the labour wards of the maternity services of the Yaounde Central Hospital (YCH) and the Gynaeco-Obstetric and Paediatric Hospital of Yaounde (GOPHY). It was conducted during a 5 month period, from 1st December 2014 to 30thApril 2015. A total of 426 Women with singleton deliveries at term with cephalic presentation were included in the study. They were classified in exposed, for those with MSAF and non-exposed, for those with clear amniotic fluid (CAF). The two groups were followed up during labour and 72 hours following delivery. Mode of delivery, foetal heart rate, Apgar score at the 1st and 5th minute, the unset of meconium aspiration syndrome and neonatal death were recorded.
Results: The incidence of MSAF was 11.15%, of which 52.1% was thick meconium stained. Maternal morbidity was high in the MSAF group with higher proportions of caesarean delivery (RR=2.35 p<0.001). Foetuses and neonates born with MSAF had higher morbidity and mortality when compared to those with CAF Complications included foetal heart rate abnormalities, low Apgar score at the 1st and 5 th minute, need for neonatal resuscitation and neonatal asphyxia. Perinatal mortality was 4.7% in the MSAF group and 00% in CAF. All cases of death (10) were seen in the thick MSAF group.
Conclusion: MSAF is associated to increased perinatal morbidity and mortality. Caesarean sections were performed twice as frequently in women presenting with MSAF.
Keywords: Meconium stained amniotic fluid; Perinatal outcome; Yaounde; Cameroon
Abbreviations: MSAF: Meconium Stained Amniotic Fluid; MAS: Meconium Aspiration Syndrome; CAF: Clear Amniotic Fluid; YCH: Yaounde Central Hospital; GOPHY: Gynaeco-Obstetric and Paediatric Hospital of Yaounde; USA: United States of America; CTG: Cardio Topography; FHR: Foetal Heart Rate
Meconium stained amniotic fluid (MSAF) is frequently encountered in obstetric and neonatal practice. Its incidence is thought to increase with gestational age, being less common in preterm labours (5%) but more common in term (7-22%) and post term deliveries (23-52%) [1-3]. Although the exact cause of this situation is not known, foetal distress, cord problems and maternal hypertension are some identified potential risk factors [4]. The passage of meconium in utero is a problem both in intrapartum and postnatal for the wellbeing of the foetus and that of the mother [5]. Studies in India and Pakistan found higher proportions of caesarean section, foetal heart rate abnormalities, meconium aspiration syndrome (MAS), low Apgar score (<7) at the fifth minute and neonatal death in cases of MSAF [6-8].In order to minimize the occurrence of these complications and improve their management, the scientific community especially in England and the USA was mobilized by setting guidelines for obstetric and neonatal management of MSAF [9-11].
These guides recommended, among others, the continuous monitoring of the foetal heart rate during labour and the use of amnioinfusion in thick MSAF. Several countries have implemented these recommendations, including the use of amnioinfusion during labour which helped to significantly reduce the rate of caesarean section and MAS [12-14]. Similarly, other practices such as the decrease in post-term births, early diagnosis of abnormal foetal heart rate, increased rate of caesarean section and early ultrasound assessment were associated with a significant decrease in MAS rate [14]. In Cameroon, literature is still poorly documented on the subject: the incidence of MSAF is not known and its consequences have not been described. This study was therefore aimed at determining the maternal and foetal consequences of MSAF so as to contribute to improving its management.
We conducted a prospective cohort study targeting pregnant women admitted in labour in the maternity services of two referral hospitals of Yaounde: the central Hospital (YCH) and the Gynaeco-Obstetric and Paediatric Hospital (GOPHY). The study was conducted during a 5 month period from 1st December 2014 to 30th April 2015.
All women with singleton pregnancies at term with cephalic presentation were included in the study. Delivery by elective caesarean section and intra uterine foetal death upon admission were not included in the study. Women were divided in two groups: exposed, for those with MSAF and non-expose for those with clear amniotic fluid (CAF). MSAF group was further categorized based on meconium consistency into thick (thick greenish meconium with particulate matter in amniotic fluid/pea soup consistency) and thin (light yellow or light green staining of amniotic fluid). Was considered as non-exposed, the first delivery with clear amniotic fluid following the exposed case.
Considering the incidence of foetal heart rate abnormality as a primary outcome in the non-exposed incidence=11% [12], the power of the study set at 80% and the error set at 5%, we obtained a minimum sample size of 150 women in each group [15].
The YCH is a referral Hospital in Yaounde, with 10 services (gynaecology and obstetrics, surgery, internal medicine, radiology, ophthalmology, oto-rhino-laryngology, emergency, laboratory, pharmacy and a mortuary). Each month, about 350 deliveries are registered at the maternity of this Hospital. The GOPHY is also a referral hospital in Yaounde, which goal is to improve quality of services rendered to mother and child in Cameroon. Its services are diversified, with an accent put on the gynaeco-obstetric and paediatrics units. It is also highly solicited by the population, with a monthly delivery rate close to that of the YCH. The current approach to MSAF management in these hospitals include continuous FHR monitoring with a CTG, though not systematic due to limited availability compared to the high demand. Amnioinfusion is not yet being performed by obstetricians, one of the reasons being the high risk of infection given the aseptic nature of our environment. Finally, non-vigorous new-borns generally have their airways suctioned after complete delivery (suctioning before delivery of shoulders is not performed), then are ventilated immediately. They can further be put on nasal oxygen therapy or tracheal intubation can be attempted by paediatricians if judged necessary.
Using a monitoring grid, participants were followed up during labour and 72 hours following delivery, checking for maternal, foetal and neonatal complications. Parameters were recorded by the main investigator, helped by 6th and 7th year medical students present in the service during the study period. Data were abstracted from the medical chart and completed by face to face interview when incomplete. An information notice about the on-going of the study was posted in the delivery rooms and the personnel briefed on the objectives of the study. The following parameters were recorded and compared between the two groups: age, parity, gestational age at delivery, number of antenatal consultations, past medical and obstetrical history, mode of delivery (vaginal, vaginal instrumental or caesarean), foetal heart rate, Apgar score at the 1st and 5th , need for neonatal resuscitation, unset of MAS and neonatal death.
Data was analysed using EPI-INFO version 3.5.4, STATA 12.1 and Microsoft Excel 2010. The incidence of MSAF was calculated by dividing the total number of MSAF cases during the study period by the total number of deliveries. Relative risk (RR) at 95% confidence interval was used as measure of association between variables in the two groups. P-value < 0.05 was considered statistically significant.
The maternal primary outcome was the mode of delivery. The incidence of each of the three modes (caesarean, vaginal and operative vaginal) was calculated in each group and compared, the Relative risk ratio and its confidence interval calculated. Foetal primary outcomes were heart rate abnormality and low Apgar score. The incidences were calculated in each group and compared, the Relative risk ratio and their confidence intervals calculated. Secondary outcomes included neonatal resuscitation, neonatal asphyxia and neonatal death. Their incidences were also calculated in each group and compared. The incidence of meconium aspiration syndrome (defined as the onset of a respiratory distress in a new born, in the context of MSAF and whose symptoms cannot be explained otherwise) was calculated in the MSAF group and compared in its sub groups(thick and thin MSAF).
The study was examined and approved by the institutional ethics review committees of the GOPHY (No 133/CIERSH/ DM/2015) and YCH (No12/2015/AR/MINSANTE/SG/DHCY/CM/ AD).Written informed consent was sought from each participant prior to inclusion in the study.
Consent for publication: Upon accessing consent to participate, consent on publication of results from the study was also obtained from the participants.
Availability of data and material: the dataset of this study will not be shared because the Ethical guidelines prohibit researchers from providing their research data to other third-party individuals.
Out of the total of 2376 deliveries registered during the study period, 265 had MSAF, giving an incidence of 11.15%. Two hundred and thirteen (213) cases of MSAF fulfilled inclusion criteria and were included in the study. They were compared to 213 cases of CAF. Fifty two MSAF cases did not meet the inclusion criteria and thus were not included in the study for the reasons presented in Table 1. MSAF was described as thick in 52.1% of cases and thin in the rest (Table 1).
Table 1: Reasons for non-inclusion of women with MSAF.
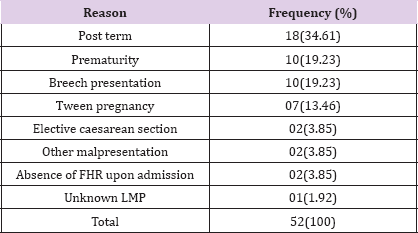
LMP: last menstrua! period; FHR: foetal heart rate
Obstetric Characteristics of the Population: The mean gestational age at delivery was significantly higher in the MSAF group (39.7vs 39.2weeks: P < 0.001). Similarly, the gestational age group of 40-42 weeks was significantly represented in the MSAF group (41.78 % vs. 26.76 %, p = 0.0011). More than half of participants (54.9 %) were multiparous. As shown in Table 2, the proportion of prolonged rupture of membranes (defined as the rupture of membranes > 12 hours before delivery) was significantly higher in cases of MSAF (Table 2).
Table 2: Obstetric characteristics of the population.
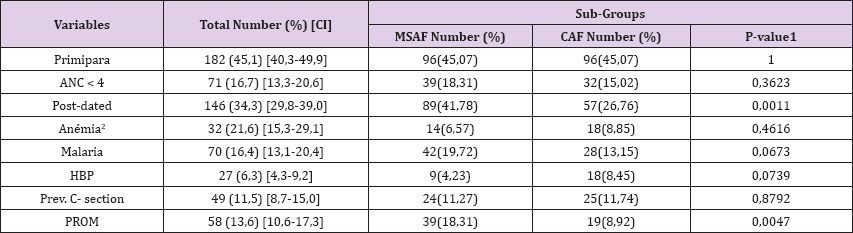
1: as determined by the Chi Square; 2: defined as haemoglobin
ANC: antenatal consultation; HBP: high blood pressure; PROM: premature rupture of membranes
Characteristics of MSAF: Among the 213 MSAF cases, 111 (52.1%) were thick and 102 (47.9%) thin. In 23.5 % of cases, the amniotic fluid was clear in early labour, then meconium stained. The majority (63.8 %) of MSAF were detected in the active phase of labour.
Table 3: Maternal morbidities in MSAF.
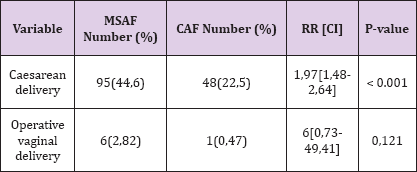
Maternal Morbidity: Among the 213 women with MSAF, 95(44, 6%) delivered by caesarean section, with the leading indication being acute foetal distress. In comparison, 48(22, 5%) women with CAF had caesarean delivery. This difference was statistically significant (RR= 1.97 P < 0.001). Similarly, the proportion of operative vaginal delivery was higher in the MSAF group, though not statistically significant, as shown in Table 3.
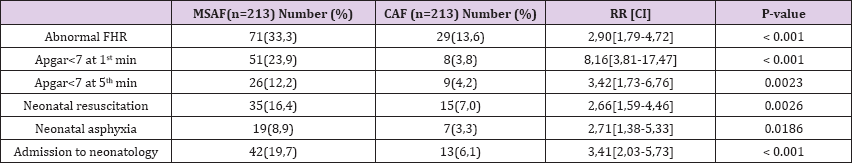
Foetal and Neonatal Morbidity: The mean birth weight was 3277.11± 493.59g. There was no significant difference of birth weight in the two groups. Meconium aspiration syndrome (MAS) was present in 5 cases (2.34%) of MSAF. The other foetal and neonatal morbidities were compared in the two groups and presented in Table 4 as follows (Table 3). The risks of low Apgar score at the 1st and 5th minute were respectively 8 and 3 times higher in the MSAF group. All the same, the risks of foetal heart rate abnormalities and neonatal asphyxia were significantly higher in the MSAF group. As seen with maternal morbidity, thick MSAF is associated with higher proportions of foetal and neonatal morbidities when compared to thin MSAF and CAF.
Table 4: Foetal and neonatal morbidities in MSAF
Perinatal Mortality: Ten (10) cases of death were registered during this study, giving a mortality rate of 2.3%. All cases of death were seen in the thick MSAF group. Five deaths occurred as consequence of MAS and five others were still births. Given that the mortality rate in the CAF group was 00%, association could not be calculated to determine whether the mortality rate was significantly higher in the MSAF group.
The incidence of MSAF was 11.15%, with 52.1% thick MSAF Mean gestational age at delivery was higher in the MSAF group (39.7weeks vs. 39.2weeks). Caesarean section rate was higher in the MSAF group (p<0.001). Regarding foetuses and neonates, MSAF significantly increased the risk of foetal heart rate abnormalities, low Apgar score at the 1st and 5th minute, admission into neonatology and neonatal asphyxia. Again, 100% (10cases) of perinatal deaths were seen in the MSAF group. The incidence of MSAF found in this study was 11.15%, which is in accordance with the range of 7 to 22% found in the literature [1-3]. Patil K et al. [13] in India found a similar incidence of 8.3%. Majority of observed MSAF was of thick type (52.1%) possibly because thin MSAF being more subjective is more prone to variations in incidence.
In spite of debate, there are still a number of unresolved controversies concerning management of the labour with meconium stained amniotic fluid. Nevertheless most obstetricians feel unsafe about the state of the foetus if meconium stained liquor is seen. This influences the mode of delivery. In setting where other facilities of intrapartum monitoring like cord blood sampling, CTG and non-stress test are not available, instrumental as well as caesarean delivery are found to be increased when meconium is present. In the present study 44.6% of MSAF group had caesarean delivery as compared to 22.5% in clear liquor group. This increase in caesarean delivery rate can be explained by the higher incidence in this same group of foetal distress (33.3% vs.13.6%); given that it is an absolute indication to caesarean delivery. Salma et al. [6] and Erum et al. [7] found similar results with caesarean delivery being three times more frequent in case of MSAF. We were in accordance with Aparna et al. [4] and Nirmala et al. [8] in India where thick MSAF is associated to higher morbidity when compared to thin MSAF or CAF.
The incidence of foetal and neonatal morbidities was higher in the MSAF group. These included FHR abnormalities, low Apgar score at 1 and 5 minutes and neonatal asphyxia. Similar results were found in India and New Guinea where FHR abnormalities were at least 5 times higher in the MSAF group [4,16]. Proportions of low Apgar score at the 1st min were 3.8, 23.9 and 36.9% in cases of CAF, MSAF and Thick-MSAF respectively. In the same order, proportions of low Apgar score at the 5th min were 4.2, 12.2 and 20.7%. These differences in proportions were statistically significant, with a higher risk in the thick MSAF group both at the 1st min and 5th minute. These results are in accordance with those found by Patil et al. [13] who had 19% low Apgar score in the MSAF group. Nirmala et al. [8] on the contrary found a low Apgar score (16% of MSAF) only at the 1st minute. Persistent difference in low Apgar score at 5th minute found in our study could be due to limited resuscitation resources in our milieu.
Meconium aspiration syndrome (MAS) was present in 5 cases (2.34%) of MSAF. Though slightly higher, Gupta et al. found a similar incidence of 4% in his series. Perinatal mortality in this study was 46.9%o in the MSAF group and 0%o in the CAF group. Moreover, all cases of death were seen the thick MSAF group. Similar mortality rates were found by Salma et al. [7] and Patil et al. [13]. This later for instance found a mortality rate of 40%o, all in the thick MSAF group. These demonstrate that MSAF and particularly thick MSAF are associated to increased risk of perinatal mortality. An important limitation to this study included limited facilities for intrapartum foetal monitoring, CTG not always being available and other methods like foetal scalp pH not vulgarized. However, findings in this study can be used to design some interventions in order to improve maternal and foetal outcomes.
Both mode of delivery and foetal outcome were affected by the presence of meconium stained amniotic fluid as compared to clear amniotic fluid. Thick MSAF is associated to higher morbidity and mortality when compared to thin MSAF and CAF. So presence of meconium should be monitored closely and additional monitoring facilities such as CTG and foetal scalp pH if available could guide obstetrician to decide the mode of delivery and any other necessary intervention on time.


