Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gottfried Lemperle*
Received: January 16, 2018; Published: January 23, 2018
*Corresponding author: Gottfried Lemperle, Division of Plastic Surgery, University of California, Wolfsgangstr. 64, D-60322 Frankfurt am Main, USA
DOI: 10.26717/BJSTR.2018.02.000682
The increasing need for long lasting injectable soft tissue fillers for the treatment of wrinkles and skin defects, gastric reflux, urinary and fecal incontinence, and other indications requires a critical discussion of biocompatibility based on science. Since biological fillers made of collagen will be absorbed over time, medium- and long-lasting biomaterials have been developed in recent years. Particles of irregular shape and rough surface structure tend to initiate severe and early foreign body reactions with scattered giant cells (“frustrated macrophages”). On the other hand, biologically inert microspheres with smooth surfaces are encapsulated by macrophages and fibrous tissue and will be kept in place. A review of the literature shows that most injected microspheres and particles of different chemical nature and a diameter of 20 to 65μm are phagocytosed by macrophages. Particles smaller than 20μm are also phagocytosed but are transported by macrophages to regional lymph nodes, liver and/or lungs. Since 1 gram of medium size microspheres of 40μm has a surface area twice as large as 1 gram of microspheres of 125μm in diameter, the stimulus for associated tissue in growth is doubled. Therefore, approximately 75% of the filler volume of a medium size microsphere implant consists of the body’s own connective tissue, whereas only 40% of the filler volume encapsulating larger beads is made up of fibrous tissue.
Abbreviations: GERD: Gastro-Esophageal Reflux Disease; PI: Propidium Iodine; PSF: Polysulfone; RES: Reticulo-Endothelial System; SEM: Scanned Electron Microscopy; PMA: Polymethylacrylate; PLLA: Polylactic Acid; PTFE: Polytetrafluoroethylene
Biomaterials have been used as implants for many decades in orthopedic, plastic, ENT, and ophthalmic surgery (Table 1). In search of an ideal soft tissue filler substance, a variety of biomaterials have been injected in form of micro particles [1] and microspheres [2- 4] beneath wrinkles, into dermal defects, in patients with gastroesophageal reflux disease (GERD) [5], and into the urethra of patients with urinary incontinence [6]. The particles and spheres differed in chemical composition, surface structure, surface charge and particle size and stimulated different host reactions accordingly.
Table 1: Classification of Biomaterials Used in Medicine.

Practically, all hard materials such as metals, ceramics and polymers, many soft materials such as silicone gel, Teflon and hydro gels, as well as biological materials such as ivory, coral, soybean oil, silk, and catgut have been used as alloplastic substitutes for human tissue or as suture materials. However, synthetic polymers made up by far the broadest and most diverse class of biomaterials used. These synthetic polymers are available with a wide variety of compositions and properties and may be fabricated readily into complex shapes and structures. In addition, their surface may be modified physically, chemically and biochemically (Table 2). Water absorption in biomaterials is very important to the function of some polymers, such as hydrogels in soft contact lenses, PMA or dextran particles.
Table 2: Injectable microspheres are used as wrinkle and bulking agents in skin depressions and stress urinary incontinence (SUI).
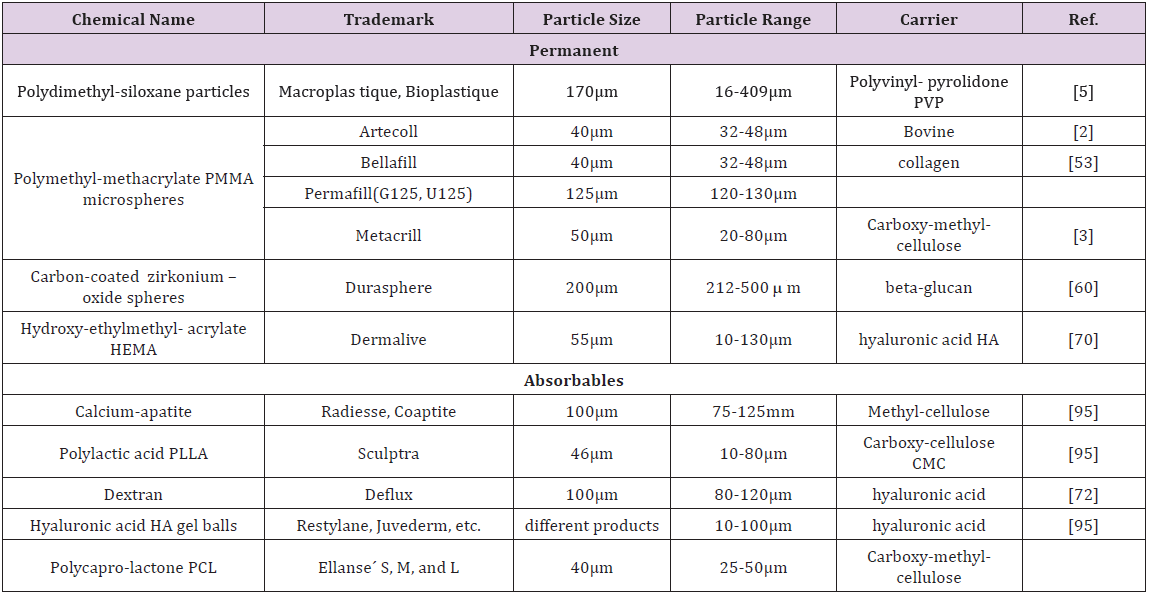
Certain water content may also lead to absorption of ions and other molecules such as enzymes, which cause biodegradation of the polymer, especially if it contains susceptible bonds [7]. Retractable sites such as –OH, -COOH, or –NH2 may be present on the polymer backbone, or may be introduced via free radical graft polymerization reaction. Such active compounds may also be electrostatically “bound” to the polymer by opposite charge or acid-base attractions of surrounding cells and fluids. In general, the behavior of particles is largely influenced by their particle character rather than by their chemical nature [8] (Figure 1). A description of the chemical nature of each particle would be beyond the scope of this review.
Figure 1: Cleaned PMMA-microspheres with apparently smooth surfaces.
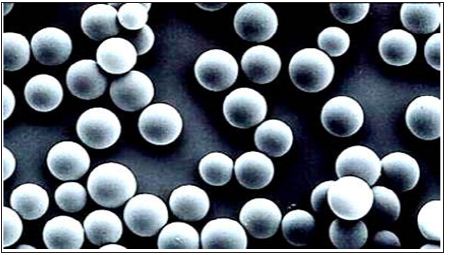
Although the chemical composition of the implant would seem to be of primary importance, its physical form is equally critical in determining biocompatibility [9,10]. A variety of physico-chemical factors affect phagocytosis, including particle size, shape, contact angles, collision factors, surface tension, and surface charge (Figure 2). A simple experiment [11] with rods of 1 mm in diameter, but different shapes implanted into rats showed that triangular polymer implants with sharp edges caused significantly higher cellular responses and acid phosphatase enzyme activity than square or round implants. Around implanted polyethylene disks with coarse surface in rats, a significant larger number of PI (propidium iodine stained) – positive cells with increased permeability were found in the tissue adjacent to the edges of the implant, when compared to implants with smooth surface [12].
Figure 2: A closer look at the surface shows many irregularities and attached small particles, which are the secret of macrophage stimulation and encapsulation.
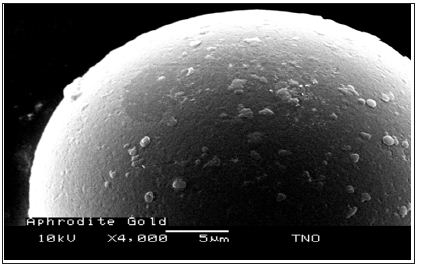
The surface topography seems to be the predominant factor relative to the induced tissue response. Taylor [9] demonstrated increased cell adhesion and increased enzyme release on textured Teflon® discs when compared to smooth Teflon® discs. The interfacial cells consisted of foreign body giant cells and macrophages in discs with rough surface, however, mainly of a fibrous capsule in smooth walled discs. At the beginning, macrophages enveloped the rough structures with inter-digitation of filopodia, frequently seen between macrophages near textured surfaces. A statistically significant difference in tissue stimulation existed also between rough and smooth particles [13]. The particles with rough surface (polysulfone PSF) were covered by foreign body giant cells, while smooth particles [2] were covered mainly by fibrous tissue (Figure 3).
Figure 3: A microsphere with an absolute smooth surface absorbs proteins and becomes slowly covered with collagen fibers. The lack of macrophage engulfment allows “migration” according to gravity.
Macrophages attach more rapidly to rough surfaces (Figure 2) than to smooth spherical microspheres [14-16]. Incubation of PMMA nanoparticles in serum, especially when complement inactivated prior to injection, reduced the uptake of radiolabeled microspheres by phagocytosis from the bloodstream into the RES [17]. Interestingly, no correlation between surface roughness and cell adhesion was noted in a study on a variety of metals and polymers [18]. Scanned electron microscopy (SEM) showed that fibroblast adhesion was highest in stainless steel and lowest in silicone rubber and polyethylene. Therefore, the composition and structure of surface molecules must play another important role in the attachment of macrophages and fibroblasts to smooth surfaces.
Microspheres from silicone, polyethylene, and tris-acryl gelatin can be produced with an absolutely smooth surface (Figure 4). They do not stimulate any foreign body reaction after injection into body tissues. On the other hand, it appears to be impossible to produce PMMA or PLLA or calcium microspheres without microscopic impurities, either attached to their surface (Figure 2) or small particles and microspheres < 20μm between the larger microspheres (Figure 5). Their number can be measured in a Coulter chamber or may be detected under a water droplet placed on PMMA-powder under the microscope (Figure 6). Impurities and smaller particles can be separated for example by wet sieving the crude bone cement powder through 2 micro-mesh sieves of 30μm and 50μm diameters. The still attached small particles can then be removed by intricate foam washing of the 40μm beads.
Figure 4: Embospheres (tris-acryl-gelatin) were found accidentally 8 years after injection into my forearm 6 cm proximal from the injection site, close to encapsulated PMMA-microspheres. There are still no macrophages attached due to their absolutely smooth surface.
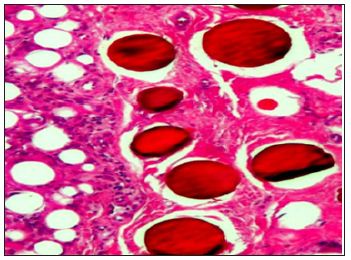
Figure 5: Brazilian PMMA-microspheres sieved from crude bone cement but not freed from small particles and microspheres <20 μm.
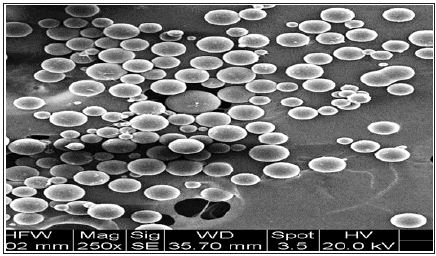
Figure 6: The focus of normal EM-pictures (Figure1) is higher than the ground: to detect impurities among the microspheres, one has to put a drop of water on the PMMA-powder, which focuses the ground.
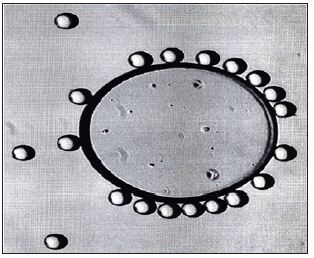
In 2006, the US-FDA requested prior to the approval of Artefill® that the content of small particles < 20μm should be less than 1%, or less than 1 particle among 100 microspheres of 40μm (Figure 1). Particles smaller than macrophages can stimulate their activity significantly because they can be transported to lymph nodes and lung – in contrast, larger particles and microspheres will remain at the injection site. Therefore, the rate reduction of foreign body granulomas associated with Artefill® and Artecoll® injections since 2006 [2] may be related to this strict and logical stipulation by the US-FDA.
All living cells and most biomaterials possess a surface charge (zeta-potential) due to their chemical composition [19,20]. This may influence cell attachment to implanted materials [18,21]. The extent of cell adhesion is dependent on the surface tension of the substrate materials (silicone, PTFE, polystyrene, polyethylene etc.). Van Waals forces are the most important factors in determining the extent of cell adhesion to polymer surfaces. Absolom [22] demonstrated a decreased number of adherent granulocytes on different polymers with decreasing surface tension. With an increase of surface tension they found less absorption of proteins, which influence adhesion of cells. in vitro studies with different polymers indicate that hydrophilicity/hydrophobicity of the implant influences the type and strength of protein bands to the material [23]. PMMA particles, for instance, have a hydrophobic surface and therefore enhance protein synthetic activity and fibroblast proliferative capacity (Figure 7).
Figure 7: PMMA-microspheres stimulate macrophage and fibroblast activity from the beginning, which holds them in place.
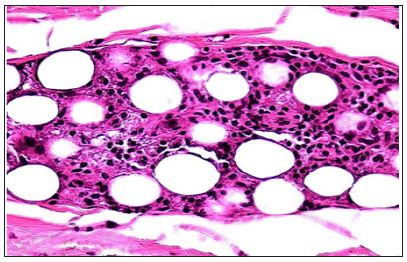
Polystyrene microspheres with hydrophobic surfaces, on the other hand, were more readily phagocytosed than those with hydrophilic surfaces [24]. There was no significant difference in phagocytosis between cationic and anionic surfaces when compared at zeta-potential of the same absolute value. The least phagocytosis was observed for cellulose and Embosphere (tris-acryl gelatin) microspheres with non-ionic hydrophilic surfaces (Figure 4). The addition of fetal calf serum to culture medium resulted in decrease in phagocytosis for all microspheres. Cell phagocytosis has frequently been noted to be similar to cell spreading on a solid substrate [25]. Cell surface charge does not vary significantly after cell death, however, dead cells exhibit different contact relations with various materials. Low-energy surfaces such as paraffin, silicone, and resins, allow only minimal cell spreading and result in a lack of adhesive bounding. Increased cell spreading observed on Teflon® particles reflects a departure of the surface energy towards the lower surface energy of the cell substrates [26].
The presence of an implant grossly changes the local dielectric environment, thus affecting local intermolecular interactions. Cells touching the implant may have their membrane potential altered due to the lack of physiological ions at the implant surface. Surface defects or lattice orientations on the implant surface can affect the tissue reaction as can residual surface charge or dipole orientation [27]. The conversion of the surface properties of metals and plastics, for example to exhibit the same critical surface tensions between 24 and 26 dynes per centimeter as natural endothelial cells, can minimize plasma protein denaturation [24]. However, little has been published on surface charges in vivo, since they are difficult to measure in living tissue [28] and may be subject to dramatic changes when in contact with surface charges of neighboring cells. On a surface with strong hydrophilic properties, an increase in cell adhesion has been demonstrated [23] in opposite to surfaces with less wetting properties. Negatively charged resin beads, such as the cation exchanger Sephadex®, induce bone formation, wound healing and giant cell invasion, whereas beads with no charges at all do not [29].
Increasing the negative surface charges of hydrophilic microspheres resulted in decreased phagocytosis [30]. Krukowski [31] implanted negatively charged dextran beads subperiosteally into rats and stimulated bone formation. Similarly applied positively charged or uncharged beads were non-osteogenic. All beads of 40–120μm in diameter were ingested with multinucleated giant cells and separated by connective tissue (Figure 8). This study demonstrates that the expression of osteogenic potential is unrelated to the physical shape of the charged particles in that both beads and particles produced new bone. However, positively charged particles were consistently phagocytosed by prominent multinucleated giant cells. Negatively or not charged beads, on the other hand, were not typically associated with such typical macrophages. It is neither clear, why positive charges preferentially attract macrophages nor clear, why and how macrophages are activated by positive charges. In tissue culture, macrophages migrate towards positively charged particles [3,4] and positively charged materials invoke a favorable wound-healing response in rat incisional wounds [32].
Figure 8: Absorbable dextran beads are surrounded by multinucleated giant cells and granulation tissue at 3 month.
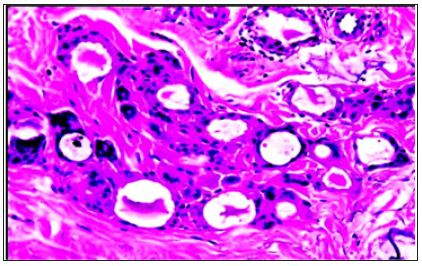
Phagocytosis, the process by which macrophages recognize and try to destroy or transport wear debris or injected biomaterials, is an essential part of host defense. According to their chemical composition and surface charge, the body reacts either with protein attachment and consequent encapsulation with fibrous tissue [33] or with an attempt to phagocytose the particles. Proteins adsorb to almost all surfaces during the first few minutes of blood or tissue exposure (Figure 3). Its amount, type conformation, and orientation of the bound proteins primarily determine the overall tissue compatibility response of the material. When exposed to foreign body surfaces, the macrophages release cytoplasmic lysosomal content (phospholipases, proteinkinase C, etc.). Intravenously injected particles are either trapped in the lungs or eliminated by the reticuloendothelial system (RES) of the liver if they have a diameter below 10μm, e.g. that of a capillary [34-37].
Macrophage activity is affected by the physicochemical state, such as particle size [26,38] chemical structure [8] and surface characteristics [39-40]. A foreign body reaction occurs when size and shape of the implant exceed the diameter of phagocytosable particles (Figure 9). In general, less severe tissue reactions have been observed with powder forms as compared to solid structures of the same material [41]. A sphere has the least surface area for a given volume. Particles with irregular shape and surface have larger and less uniform surfaces, therefore providing more stimulation to the macrophage and initiating release of cytokines. Furthermore, large particles with rough surfaces, which cannot be phagocytosed produce more inflammation. (Figure 9) An increase in surface area allows for increased protein binding in the form of IgM or IgG, leading eventually to increased release of cytokines [15].
Figure 9: Subdermally injected silicone particles (Macroplastique®) stimulate a typical foreign body reaction with giant cells (“frustrated macrophages”) at 6 month.
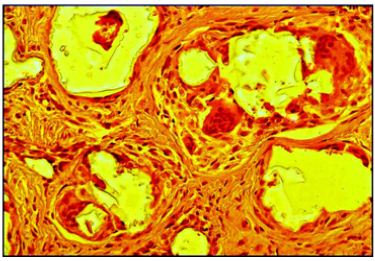
Macrophages, histiocytes or phagocytes have an average diameter of 15 to 20μm. In tissue cultures as well as in living tissue the phagocytosable size of particles is less than 5μm [42]. However, in clinical situations macrophages are able to phagocytose particles and microspheres up to their own size of 15μm and even bigger. Exceptions with phagocytosis of larger particles have been seen occasionally by the formation of multinucleated giant cells [43], which cannot ingest the foreign body by themselves (Figure 9). This unhealthy appetite will cause the death of these converted macrophages with consequent release of intracellular enzymes.
Phagocytosis so far has been described for particles up to a size of 15μm [44]. A raw surface was the prerequisite [44,45]. In experiments with mouse peritoneal macrophages [26] the maximal phagocytosis of polystyrene microspheres took place when their size was in the range of 1.0–2.0μm. In tissue cultures human leucocytes were not able to eliminate polystyrene spheres with a diameter greater than 7μm by phagocytosis [45]. If larger particles are a stimulus for phagocytosis, the macrophage increases its size and number of nuclei and converts to a multinucleated giant cell (Figure 9). These giant cells can reach a size from 40μm up to 150μm and contain up to 40 nuclei [3].
Eppley [4] calls a giant cell a “frustrated macrophage”, since it is not able to transport the foreign material to lymph nodes or liver, even if it was able to ingest it. Because it is not possible for a macrophage of 20μm to transport a 40μm particle, “migration” of these bigger particles can be provided only by mistakenly intravascular injection [46]. In tissue culture, giant cells amass cytoplasm up to a diameter of 150μm by 10μm [47], but are unlikely to be capable of migration. There is a vast amount of orthopedic literature on wear particles from PMMA bone cement or artificial silicone joints, transported to axillary or inguinal lymph nodes. It is generally agreed that small particles of less than 12μm are a critical factor associated with bone resorption [48].
Gonzales [49] compared phagocytosable PMMA particles (0.325 and 5.5μm) with non-phagocytosable (200μm) spherical particles. Although macrophages appeared to adhere to the big particles, hexosaminidase and PGE2, both markers of macrophage activation, were not stimulated. Therefore, we have to assume that macrophages stop their biological activity as soon as they are full and unable to move. Since the life span of macrophages is very short, i.e. two days only, a constant turnover of these cells must occur within foreign implants. Only phagocytosable PMMA-particles stimulated peritoneal macrophages to secrete interleukin-1 and PGE2 [51]. PMMA-microspheres enhanced human fibroblast proliferative capacity and protein synthesis in vitro. In a test series with PMMA-microspheres of o.6μm, 2.0μm and 4.5μm in diameter, cell death occurred only after phagocytosis of 1,250 of the smallest microspheres per cell [51]. The largest PMMA particles from crushed bone cement phagocytosed in-vitro by macrophages were 12μm in diameter. These macrophages released TNF alpha and showed a decreased H3-thymidine uptake compared to inactive cells [48].
Interleukin expression and increased TNF expression was also described by Herman [52] after phagocytosis of PMMA microspheres of 1–2μm in diameter. Gelb [15] showed increased TNF expression after phagocytosis of small PMMA particles of less than 20μm in diameter in a rat´s air pouch simulating synovia. Irregular particles produced a greater response in TNF and PGE2 expression than spherical particles of the same size. Particles of 50-350μm in size caused no expression of these factors. PMMA microspheres of 32-40μm in diameter with tiny impurities (Figure 2) on its surface injected subdermally into rats [53] caused a mild foreign body reaction and encapsulation with fibrous tissue within the first weeks (Figure 7). No “migration” of microspheres could be detected in adjacent lymph nodes or distant organs [54,55].
Implantation of washed PMMA microspheres of 32-40μm in diameter with reasonable smooth surface [56,57] caused rarely foreign body reactions [57,58] but immediate phagocytosis, encapsulation with fibroblasts and collagen fibers in humans. Three months after implantation of PMMA-microspheres, one can detect in-growing capillaries, which convert the injectable into a “living implant”, which bleeds when cut (Figure 10). Human macrophages of the cell line U 937 phagocytosed high numbers of small PMMAmicrospheres of 4μm and 8μm in diameter, but one microsphere of 20 or 40μm in diameter [59].
Figure 10: PMMA-microspheres are all encapsulated by macrophages and show in-growing capillaries at 3 month. They have created a “living implant”.
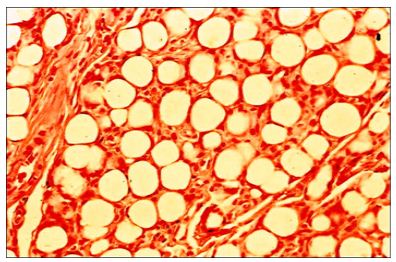
The diameter of the microspheres plays an important role in determining the amount of fibrous tissue stimulated by the injectable implant. From histological pictures (Figure 4) one can assume that each bead is covered outside its ingesting macrophages by a fibrous capsule of about 10μm in width. If one calculates the volume of beads against the volume of connective tissue in a certain volume of implant, roughly 75 % of the permanent volume with 40μm beads consists of the body’s own fibrous granulation tissue, whereas the stimulated fibrous tissue around 100μm beads accounts only for approximately 50% of the permanent implant (Figure 11).
Figure 11: The calculated volume of connective tissue surrounding PMMA-microspheres depends on the size of their size: as bigger they are, as less tissue they generate.
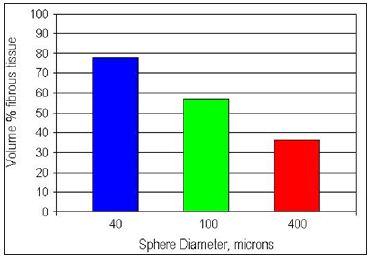
Permanent PMMA-microspheres stimulate an average of 80% of granulation tissue within the first year (Figure 12), which then converts into pliable scar tissue with predominantly collagen fibers produced by scattered fibroblast. The microspheres are still engulfed – or better: covered - by extremely stretched macrophages, which keep them in place. Multinucleated giant cells cannot be detected anymore (Figure 13). A former transurethral bulking agent, Durasphere® [60], consisted of carbon coated zirconium-oxide beads of 212–500μm in diameter. An 18G needle is necessary in order to deliver 2.5–7.0 cc of these relative big black macrospheres into the delicate periurethral tissue. Another material of microspheres of 120-355μm in diameter, with smooth surface was Bioglass® [61]. Injected around the bladder neck of pigs, no inflammatory response, no foreign body reaction, or migration was detected of the glass beads. However, this little macrophage activity and only tender encapsulation with soft tissue (Figure 4) was the obvious reason for dislocation according gravity in form of a prolapsed [62].
Figure 12: The carrier collagen or carboxy-methylcellulose is absorbed within the first week and leaves the injected PMMA-microspheres packed in the tissue. Slow ingrowth of granulation tissue pushes them apart until a lasting “living implant” is developed.
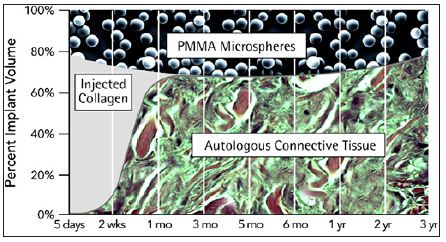
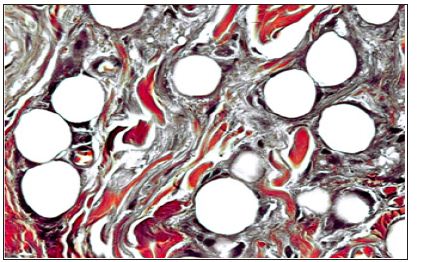
Figure 13: Old PMMA-implant with predominant collagen fibers, scattered fibroblasts and absent giant cells at 10 years. Every microsphere is still enveloped by one or more macrophages.
Silicone particles up to a size of 60μm from wear of a temporomandibular disc were engulfed by giant cells and found in the joint-capsule [63]. After implantation of Swanson® finger joint prostheses, silicone particles up to 65μm were found in the adjacent synovia [64] and up to 45μm particles in giant cells of axillary lymph nodes [65]. Fifteen years after implantation of Swanson® silicone prosthesis for metatarsal-phalangeal joint replacement silicone particles up to a size of 24 to 40μm in diameter were found in an enlarged lymph node of the groin [66]. Of course, these particles or microspheres did not migrate actively, i.e. swim through lymph vessels to lymph nodes but were actively transported by macrophages through lymph or blood vessels [55].
Davis [67] demonstrated the non-toxic nature of PMMA particles 1–100μm in diameter from bone cement (Simplex®) in tissue cultures. Only irregular particles up to a diameter of 50μm were phagocytosed [68]. Small particles less than 5μm had a greater stimulatory effect than particles of non-phagocytosable size (21–85μm) when the same concentration or same number of particles (polystyrene or titanium) was used. Particles larger than 20μm had very little or no stimulatory effect as measured by H3- thymidine uptake or cytokine release. In general, many articles on bone cement discuss the chemical inertness and biocompatibility of PMMA [69,70].
Phagocytosis and transport to distant organs has never been reported for Macroplastique® [47], a suspension of 40 % silicone particles in Povidone® PVP, which caused severe granuloma formation in the skin (Figure 9) but less around the urethra. The irregular particles range from 16–400μm and 7 % are less than 50μm [71]. The lesser stimulatory effect of Macroplastique® on foreign body reactions in and around the urethra is certainly due to the highest immunological activity in in the skin compared to internal organs.
A suspension of 50 % dextran beads of 80–120μm in diameter in hyaluronic acid (Deflux®) implanted into the bladder of rabbits demonstrated no distant migration, however [46]. These dextran microspheres caused severe granuloma formation [32,72] until they are resorbed after 1 to 2 years (Figure 8). Burgess [73] used positively charged dextran beads of 40–120μm in diameter for the enhancement of wound healing. Macrophages and multinucleated giant cells were found contiguous to the beads, but the authors doubt that they are engulfed (Figure 8).
In general, particles of biomaterials with rough surface like Teflon® [43], silicone polymer [58], ceramics [74] and those particles with a diameter greater than 20μm cannot be phagocytosed by normal macrophages. Instead, they initiate the formation of a foreign body granuloma. After the implantation of these particles, mononuclear cells from the blood infiltrate the foreign body. Then, mitotic proliferation of cells in blood-vessel walls is seen, followed by an invasion of histiocytes and macrophages. These cells have a short turnover rate of a few hours and phagocytose acceptable particles and bacteria within minutes. Cells laden with particulate matter do not divide, but when they die, their cytoplasmic inclusions are taken up by young histiocytes.
Probably, lymphocytes, which are often called intermediate small round cells, are the progeny of histiocytes [75] and macrophage. The presence of plasma cells indicates that in addition to inflammation an immune response occurs [74]. If particles are too big for phagocytosis by mononuclear cells, these fuse with other macrophages [75] or convert into multinucleated giant cells [4], which are the predominant cell type of a granuloma (Figure 14). Focal aggregates of histiocytes remain active and occasionally epitheloid cells are seen [76]. Giant cells, in general, have a life time of only approximately 1 week [10]. The reaction of the body to the same synthetic may be more or less pronounced in different organs. Immunological reactions are considerable higher in the dermis [77] and subdermis than in bone and muscle [70].
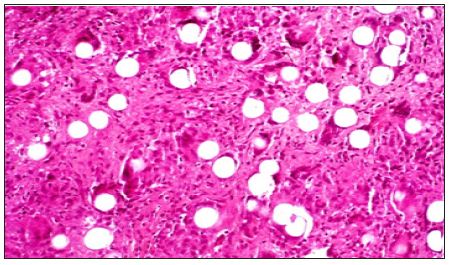
Figure 14: Typical PMMA-granuloma: the microspheres are pushed apart by suddenly invading granulation tissue containing great numbers of macrophages and giant cells. This overreaction can be treated effectively with intralesionally injected corticosteroids [80,81].
Depending on their chemical structure and surface characteristics, most absorbable biological materials and synthetics such as suture materials [78], alginate microspheres [79], polylactic acid, dextran or polymethylacrylate (PMA) particles (Table 2) initiate a temporary foreign body reaction which may remain untreated up to 12 months; whereas granulomas after permanent injectables like PMMA can last up to 5 years [80,81]. Polylactic acid (PLLA) particles of a mean diameter of 60μm, 1.0 mm and 2.2 mm respectively, were implanted subcutaneously on the back of rats [82]. They were surrounded by young fibrous tissue from 3 weeks up to 50 weeks and showed fragmentation and phagocytosis by macrophages and foreign body giant cells after 70 weeks of implantation [82]. If implanted intradermally, this reaction may clinically be much more obvious than when implanted subdermally or periurethrally (Figure 15).
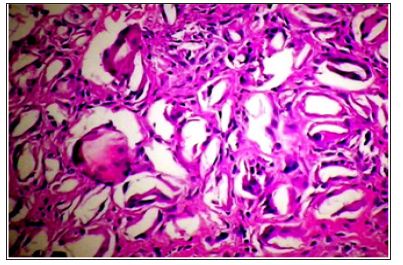
Figure 15: All absorbable particles and microspheres - here from PLLA at 6 month - are broken down by tissue enzymes, macrophages and most often giant cells over time. Their clinical effect lasts between 6 and 12 months.
Migration of particles is a main issue of artificial joint replacement surgery as well as of injectable bulking agents in urology. In this context, however, migration appears to be misnomer. Particles of biomaterials cannot actively migrate within the body, but have to be phagocytosed by macrophages migrating to lymph nodes or liver, respectively. When trapped in the lungs, they have to be injected accidentally into a venous plexus at the injection site [82]. Of course, extracellular “migration” of particles by mechanical forces such as muscle movement, skin folding, and gravity is a well-known phenomenon. Since it implies no active movement of particles, it should be called correctly “dislocation”.
With the exception of injected silicone oil in a very loose skin or gel from ruptured breast implants, which can be pushed along muscle compartments to elbow or groin, the passive mechanical dislocation of microparticles is generally confined to a distance of a few mm. McClelland et al. [54] observed “transepidermal elimination” of PMMA microspheres in guinea pigs. Since this phenomenon was seen on day 3 it must have been due to intraepidermal implantation of the material.
A widely quoted article appeared by Malizia [43], who injected Polytef® (PTFE) paste along the entire length of the urethra through an 18G needle in dogs and monkeys. Polytef® paste is composed of irregular particles, 90 % being between 4 and 40m in diameter and 70 % under 20μm. Besides granulomatous reactions in pelvic lymph nodes, granulomas were detected in the lungs and other organs, astonishingly, not in the liver. The main particle size in distant organs was 4 to 20μm, however, “their greatest dimension ranged from 4 to 80μm”. This sole mention of an 80μm long, irregular PTEF particle in the lung caused the manufacturers of bulking agents for urinary incontinence to produce particles or microspheres larger than 100μm in diameter, which was designated the “critical particle size” (5,6).
The diameter of the venous plexus around esophagus and urethra is around 80μm, which would allow falsely injected smaller particles or microspheres to swim directly to the lung [6]. The usual migration of macrophages with phagocytosed particles is towards lymph nodes or liver. Knowing the large venous plexus surrounding the urethra, it was suspected that Polytef® must have been injected in part intravenously to be taken up by the lungs [81]. Otherwise, the presence of a particle of 80μm in length within a granuloma in the lung cannot be explained. There is absolutely no proof of the statement by Henly [82] that”particles smaller than 80μm have a tendency to migrate”. Migration within the body is usually an active process performed by macrophages and exceptionally giant cells, not by inert particles.
Henly [82] injected large (32–270μm) and small (11–160μm) PTFE particles periurethrally in dogs. He found small particles in lymph nodes, lungs, kidneys and brain surface, however, not in liver and spleen. In the lung of one dog, he detected one particle of 65μm length. No particles were found in any of the tissue specimens in the group, which received large (32–270μm) particles. All particles surrounded by giant cells in the lungs were blood-born substances injected into small veins and trapped in the first capillary bed encountered [47,81]. For the purpose of embolization, PMMAmicrospheres of 1 mm in diameter were injected intra-arterially in patients with intracranial vascular tumors [83]. They did not elicit any inflammatory reaction inside vessels. Dewan [84] described a woman who experienced a skin rash and polyarthropathy 10 days after the removal of a pelvic mass containing a PTFE granuloma of 10 cm in diameter. Skin biopsy revealed vasculitis containing foreign body giant cells with refractile material consistent with PTFE.
Particulate material may embolize after accidental intravenous injection or intra-operative intrusion into the venous system [86]. Dewan [85] injected PTFE and silicone particles (Macroplastique®) into rats, but found no foreign material at distant sites after 3 and 6 months, however, a marked local foreign body granuloma reaction was noted (Figure 9). Claes [86] found PTFE particles in the lungs after periurethral implantation. The rapid onset of symptoms and the apparent volume of paste “immigration” indicated that the injection had been in part intravenously. If one looks at the diameter of the urethral venous plexus and imagines a 21G needle with a diameter of 0.8mm, intravenous injection appears easily explained. A deadly fat embolism 8 hours after periurethral injection of autogenous fat has been reported. At necropsy many occluded periurethral veins were found [87].
Steyaert [88] described the case of a nine year-old girl whose left kidney had to be removed. At the age of 18 month, a left stage III ureteral reflux had been treated with 0.5g of Polytef®. After surgery, the Polytef® could not be detected by ultrasound at the orifice of the ureter and stage III reflux remained. Seven years later, nearly all of the initial 0.5g injection was found in a large granuloma occupying the lower pole of the left kidney. One explanation for this event is inadvertent injection into the periureteral veins, which flow into the venous plexus of the renal hilus. A frightening number of <100 cases of sudden blindness occurred after accidental injections of collagen, dermal fillers, and fat [90] into the nasolabial and supratrochlear artery. These arteries were filled retrograde and connected with the central retinal artery, which was also occluded.
Plastic materials have been implanted as solid and as injectable devices. Many studies have investigated the risk of malignancies associated with the implantation of foreign materials in rodents and found that the tumors developed in the capsule around large implants [91] or sheets [92]. However, it has been suggested, that the risk of malignancy is reduced when the implanted foreign body is perforated, and virtually eliminated if the implant is a powdered polymer [41].
Theoretically, sarcomas may arise from chronic granulomatous inflammation as it is caused by irregular particles (Polytef®, Macroplastique®), foam rubber (polyurethane), or f.i. “textured” breast implants. After silicone fluid injections into rats, 8 % of the animals developed sarcomas [93]. However, no such an event has been reported in humans. The rat is known to have a high incidence of tumor formation when foreign material is implanted under the skin of the back [92,94]. The only carcinoma developing in humans with a chronically inflamed, non-healing wound, is Marjolin’s ulcer. The present literature on injectable particles and microspheres used for cosmetic purposes [95], bulking agents [96], drug delivery [97] or radiation therapy does not reveal any correlation with or publication on possible tumorigenesis.
The biocompatibility of implant materials is based upon the “fibrous capsule” that envelops the implant. This capsule represents the end stage of a repair process, which begins when plasma proteins contact and adhere to the material during implantation. Various proteins act as chemotactic factors for the infiltration of neutrophils and macrophages [98]. The types of cells present or absent from the interface characterize the nature of the reaction. For example, the absence of lymphocytes at the material interface and from the perivascular region of nearby capillaries implies that the polymer does not elicit an immune response [99].
The late development of massive giant cell formation around an implant must be considered a foreign body granuloma. This has been defined by Spector [75] as giant cells surrounded by palisading macrophages enveloped in a halo of lymphocytes. Macrophages and multinucleated giant cells are the dominant characteristics of the long-term biological response to rough surfaces and particles with irregular shape and surface. Both cell types eliminate foreign body material from the tissue. Since most of the injected irregular particles are too big for phagocytosis and subsequent transport, a chronic rejection process is set in motion, which lasts until the implant is removed.
In contrast to the rough and irregular surface, a monolayer of macrophages surrounded by a zone of fibrous tissue is found at the surface of a smooth walled implant or an absolute smooth microsphere. This is the sign of optimal biocompatibility. These microspheres are enveloped with fibroblasts, which remain in a steady state with the implant for the rest of the recipient’s life (Figure 3). It seems as if substances with medium size microspheres are able to create the best tissue augmentation in form of fibroblasts and collagen fibers surrounding the microspheres (Figure 10 & 13), thereby consolidating the bulge implanted beneath the skin, the urethral or esophageal epithelium, respectively.


