Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Birgitte Brandstrup*
Received: December 29, 2017; Published: January 10, 2018
Corresponding author: Birgitte Brandstrup, Consultant Surgeon, Department of Surgery, Holbaek University Hospital, Holbaek, Denmark
DOI: 10.26717/BJSTR.2018.02.000647
In the western part of the world, cancer in the colon and the rectum is the third most common cancer with an incidence of 1.4 million new cases in 2012 [1]. Accordingly, the number of colonoscopies performed on suspicion of cancer is large and because of the initiation of screening programs for blood in the stool also increasing. The screening programs for colorectal cancer have downgraded the majority of the tumors found from the cancer stages T3-4 to T1-2 [2,3]. And thus increased the number of potentially endoscopically removable T1 tumors. In addition, the demography of the population is changing, with a greater number of elderly patients with greater probability of having co-existing diseases. The medical co-existing diseases play a major role in the risk of adverse outcomes including death following the surgical resection of the colon or the rectum irrespective the procedure is performed laparoscopically or by open surgery [4-6]. At the same time, endoscopist’s are becoming increasingly skilled in the removal of large colorectal polyps by the technique of endoscopic mucosa resection (EMR) or endoscopic mucosa dissection (ESD).This leads to two problems: What are we going to do, with the patients who have early cancer in a polyp and how are we going to treat the patients suffering perforation during the endoscopic procedure?
Even skilled endoscopes’ have a problem judging the adenomas benign or malignant by the eye, and other methods have also shortcomings. Biopsies will often be negative for cancer even when cancer is present [7], and many polyps judged benign by the lifting technique is in reality T1 tumors [8]. Even though large polyps more frequently harbor dysplastic- or malignant cells, malignancy can sometimes be found in small lesions as well [9]. However, the risk of lymphatic spread depends on the invasion of the submucosa layers and the growth of the tumor [10-12]. Tumor growth in the most superficial layer of the submucosa (SM1) holds about 4% risk for lymphatic spread while growth to the intermediate layer (SM2) has a risk of 8%. Given a PET-CT-scan without active lymph glands in the tumor area, the endoscopic removal may therefore be sufficient. In Denmark, surgeons also perform the colonoscopies, and the patient’s path from endoscopy to the MDT conference and the operation room is therefore very short.
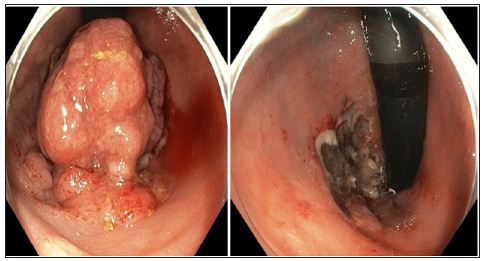
This lead to a revision of the recommendations from the Danish Colorectal Cancer group [13]. With refinement [14]. Simple endoscopic polypectomy may treat stalked polyps with a Haggit’s level 1-3 cancer [10]. And the EMR procedure may be considered definitive treatment in flat or broad based low risk cancers [11]. Especially if the patient has severe co-morbidity. There is no evidence in the literature that the EMR procedure influences the patient’s prognosis in any negative way. In our clinic, we have a series of patients with endoscopically removed cancers, both by EMR and by simple polypectomy. The majority of the patients have been free of recurrence, but a few has had local recurrence and has undergone surgery. Figure 1 shows a 60-year-old woman with a tumor in the rectum before and after EMR. The tumor had biopsies with low-grade neoplasia but turned out to be T1, SM1. We have until now followed her with endoscopy, rectal ultrasound examination (initially MR) as well as PET-CT for 3.5 years and she has no recurrence.
Figure 1: T1 tumor, Kikuchi level SM1, before and after endoscopic resection (EMR). The small area marked with an arrow was removed after the picture was taken.
With the endoscopic treatment of large polyps comes the problem with handling the complications to the endoscopic resection. However, the risk of perforation is low, and the evidence for treatment of iatrogenic perforations with either surgery or endoscopy based on reports of cases or series of patients. The traditional way of handling a colorectal perforation is surgery. Evidence increases, however, that endoscopic clips can safely close iatrogenic perforations followed by antibiotic treatment in the surgical ward [15,16].
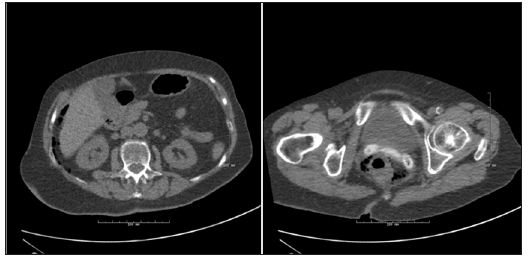
In our ward the experiences with endoscopic closure is good, even after large lacerations. Figure 2 shows an82-year-old woman undergoing flexible sigmoidoscopy for rectal prolapsed. The perforation occurred during retro flexion of the scope, and was located in the upper part of the rectum. Normal hemo-clips closed the lesion within 2 hours after the injury and she received antibiotics intravenously for 3 days. She was discharged in habitual condition with no complications at follow-up. During the past 4 years, surgical closure has only treated late recognized perforations often caused by thermal injury in our institution. Perforations recognized during the endoscopic procedure have been closed endoscopically, but like in open surgery, it is important that the endo-clips are including the sero-muscular layer of the intestine. In conclusion, the MDT-conference must decide the choice of treatment for each patient individually. The risk of adverse outcomes of surgery must balance the risk of lymphatic spread of the tumor removed endoscopically especially in elderly patients with co-morbidity. The above examples illustrates, and the literature supports, that advances in endoscopic surgery can supplement and in some cases replace conventional surgery for the benefit of the patients [17,18]. For both patients shown here, the alternative surgical procedure at best would result in a permanent stoma. A situation many of us would risk much to avoid.
Figure 2: CT-scan showing perirectal- as well as intraabdominal air. The looping of an endoscope caused the 1.5 cm perforation. Eleven haemo-clips successfully closed the defect.
The Regional Committee of Research; The Region of Zealand, Denmark and The Department of Surgery, HolbaekUniversit Hospital, Holbaek, Denmark.


