Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
S Muralikrishna*, P Jagadeeswara rao and P Ravisankara reddy
Received: October 10, 2017; Published: December 12, 2017
Corresponding author: S Muralikrishna, Santhiram College of Engineering & Technology, Nandyal-518501, A.P, Biological E Ltd Company, shameerpet, Hyderabad, India
DOI: 10.26717/BJSTR.2017.01.000582
A series of some novel 2-[5-(substituted phenyl)-[1,3,4]oxadiazol containing 1H-indazole moiety were synthesized by using of indazole with mannich base on reaction to give 3-(piperazin-1-yl)methyl)- 1H-indazole which is turned into Ethyl 2-(3-piperazin-1-yl)methyl)- 1H-indazole-1-yl) acetate. The reaction with hydrazine hydrates in ethanol solvent under reflux. The subsequent treatment of 2-(3-((piperazin- 1-yl) methyl)-1H-indazole-1-yl) acetohydrazide, with an appropriate aromatic carboxylic acid in presence of polyphosparic acid under reflux afforded the title compounds. The chemical structures of the newly synthesized compounds were elucidated by their IR, 1H NMR and Mass spectral data analysis. Further the compounds are used to find out their ability towards anti microbial and nematicidal activity.
Keywords: Antibacterial activity; Antifungal activity; 1H-indazole; PPA; Mannich base; Oxadiazole
Recent drug discovery studies have focused on the design and synthesis of small molecules that have a 1H-indazole nucleus as the core structure and that act as tubulin inhibitors [1]. Drugs that bind to tubulin act by interfering with the mitosis of cells during the M-phase, resulting in mitotic arrest and eventually lead in to apoptosis [2]. Therefore, microtubules are a sensitive target for the development of anticancer drugs. Due to the introduction of vinca alkaloids such as vincristine and vinblastine for the clinical therapy of cancer, 1H-indazole carrying compounds have generated considerable interest [3-8]. A large numbers of synthetic 1H-indazole-containing drugs and clinical candidates have been identified over the past few years Chang and co-workers reported a large number of compounds with 1H-indazole core structure. In addition to the synthesis and evaluation of the anticancer activity of these compounds, they have revealed some SAR and pharmacophore modeling data [4,5,9-13]. Research on 1- and 3-aroylindoles9 showed that 3-substituted 1H-indazole derivatives exhibited significant activity compared with 1-aroyl1H-indazoles and the electronic effects on the 1H-indazole ring were important for activity potency [11].
The oxdiazole chemistry has been developed extensively and is still developing. Presently there are a number of drugs used clinically, which comprise oxadiazole moiety in association with various heterocyclic rings. 1, 3,4-oxadiazoles are biologically active, synthetically useful and important heterocyclic compounds. The synthesis of novel oxadiazole derivatives and investigation of their chemical and biological behavior have gained more importance in recent decades for biological, medicinal and agricultural reasons. Different classes of oxadiazole compounds possess an extensive spectrum of pharmacological activities. Differently substituted oxadiazole moiety has also been found to have other important activities such as antibacterial [12], antimalarial [13], antiinflammatory [14], antifungal [15], anticonvulsant [16], analgesic [17], antimicrobial [18], antimycobacterial [19], anticonvulsant [20], antitumor [21], antimalarial [22], herbicidal [23], vasodialatory [24], cytotoxic [25], hypolipidemic [26] ulcerogenic [27] (Figure 1) and (Table 1).
Figure 1:
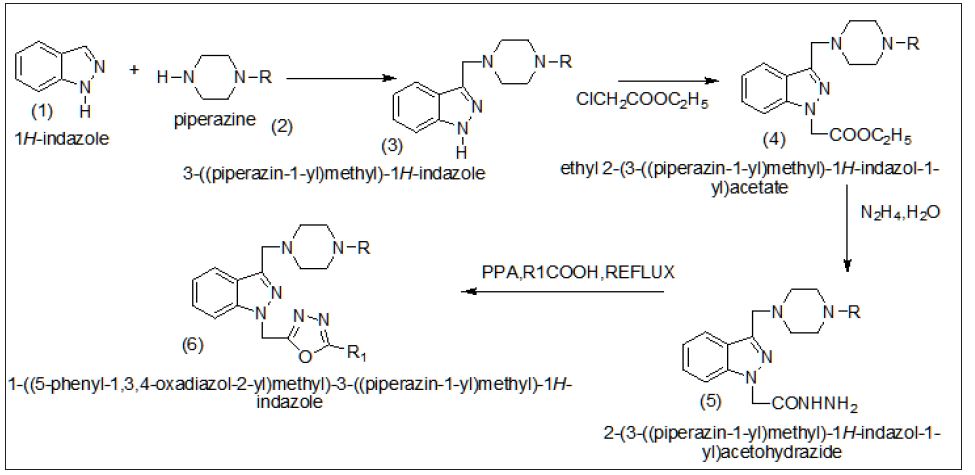
Table 1:

Chemicals and reagents used in the current study were of analytical grade. The reactions were monitored by thin layer chromatography (TLC) on Merck pre-coated silica GF254 plates. Melting points were determined using a Mettler Toledo FP62 capillary melting point apparatus (Mettler-Toledo, Greifensee, Switzerland) and were uncorrected. Infrared spectra were recorded on a Perkin-Elmer Spectrum One series FT-IR apparatus (Version5.0.1) (Perkin Elmer, Norwalk, CT, USA), using potassium bromide pellets; the frequencies were expressed in cm-1. The 1 H- and 13C-NMR spectra were recorded with a Varian Mercury-400 FT-NMR spectrometer(Varian, Palo Alto, CA, USA), using tetramethylsilane as the internal reference, with chloroform- CDCl 3 as solvent, the chemical shifts were reported in parts per million (ppm) and coupling constants (J) were given in hertz (Hz). Elemental analyses were performed on a LECO 932 CHNS instrument (Leco-932, St. Joseph, MI,USA) and analyses for C, H, and N were within } 0.4% of the theoretical values.
1H-indazole (1) (2 mmol, 235 mg) was dissolved in 20 ml of ethanol-water (1:1) solution, and formaldehyde 37% (3mmol) and substituted piperazine (2) (2 mmol) were added. The mixture was stirred at room temperature and the reaction was controlled by TLC in benzene: methanol (9:1) and toluene: ethyl acetate: diethylamine (75:25:1).At the end of the reaction, the precipitate was filtrated, dried, and recrystallized using an appropriate solvent. Yield: 45%: mp 179.7 ◦C. IR (KBr) cm-1: ν 3130 (N-H), 3095-2756 (C-H). 1H-NMR (CDCl3): δ 8.10 (bs,1H, 1H-indazole N-H), 7.77 (d, 1H, indole H4 , J = 7.6), 7.36 (d, 1H, 1H-indazole H7 , J = 8), ,6.92- 6.82 (m, 3H, 1H-indazole H2, H5, H6), 3.79 (s, 2H, C-CH2 -N), 3.20 (t, 4H, piperazine H3, H5 , J = 4.8), 2.68(t, 4H, piperazine H2, H6 , J = 4.8). Anal Calc.: C, 77.35; H, 7.35; N, 14.42%, found: C, 78.16; H, 6.94; N, 14.25%
An equimolar mixture of 3-(piperzin-1-yl) methyl)-1H-indazole (3) and chloro ethyl acetate were dissolved in dimethyl formamide solvent and to this reaction mixture anhydrous K2CO3 was added and the reaction mixture was stirred at room temperature (350C) for 8 hours and the progress of the reaction was monitored by TLC using cyclohexane and ethyl acetate solvent mixture (7:3) as eluent the reaction mixture was kept overnight. After completion of the reaction the solvent was evaporated on rota-evaporater. The gummy solid was separated and it was recrystallized from -2-propanolpetrolium ether (800c) solvent mixture. The crystalline solid was found to be -2-(3-formyl-1H- 1H-indazole) acetate. With a yield of 75% and mp 143-1450C.The indole-3-carbaldehyde used in the present studies was purchased from Aldrich Company and was used without any for their purification. Yield 75%, m.p.:143-1450C.
Yield: 55%: mp 185.7 ◦C. IR (KBr) cm-1: ν 3150 (N-H), 3095- 2782 (C-H). 1H-NMR (CDCl3): δ 7.60 (d, 1H, 1H-indazole H4 , J = 7.6), 7.20 (d, 1H, 1H-indazole H7, J = 8), ,6.95-6.85 (m, 3H, 1H-indazole H2, H5, H6), 3.85 (s, 2H, C-CH2 -N), 3.25 (t, 4H, piperazine H3, H5 , J = 4.8), 2.70(t, 4H, piperazine H2, H6 , J = 4.8), 1.29 (t,3H, J=13.2Hz, CH3 of ethyl group), 4.13 (q, 2H, J=13.2Hz, CH2 of ethyl group),. Anal. Calc. for: C, 78.32; H, 7.26; N, 14.42%, found: C, 78.18; H, 6.70; N, 14.15%,
A solution of 4 (0.01mol) and hydrazine hydrate (0.015) in ethanol (20ml) was refluxed for 5 hours. The reaction mixtures was cooled and poured in to ice-cold water with stirring. The separated solid was filtered, washed with water and recrystallized from ethanol.
Yield: 50%: mp 180.7 ◦C. IR (KBr) cm-1: ν 3160 (N-H), 3070- 2780 (C-H). 1H-NMR (CDCl3): δ, 7.65 (d, 1H, 1H-indazole H4 , J = 7.6), 7.35 (d, 1H, 1H-indazole H7 , J = 8), ,6.80-6.85 (m, 3H, 1H-indazole H2, H5, H6), 3.80 (s, 2H, C-CH2 -N), 3.25 (t, 4H, piperazine H3, H5 , J = 4.8), 2.70(t, 4H, piperazine H2, H6 , J = 4.8), ,4.28(s,2H,-NH2), ). 4.36 (s, 2H N-CH2-C =O), 4.98 (s,1 H,-N-NH), Anal. Calc. for: C, 78.32; H, 7.26; N, 14.42%, found: C, 78.18; H, 6.94; N, 14.25%.
A mixture of 2-(3-((piperazin-1-yl) methyl)-1H-indol-1- yl) acetohydrazide (5) (0.01 mol) and substituted carboxylic acid (0.01 mol) was heated at 100-120 oC in presence of excess polyphosphoric acid (PPA) for 4-5 h. After cooling, the mixture was poured into crushed ice, and neutralized with 5% aq.NaHCO3 solution. The precipitated solid was filtered and purified using column chromatography (petroleum ether: ethyl acetate, 9:1).
Yield: 60%: mp 190.7 ◦C. IR (KBr) cf-1: ν 3150 (N-H), 3050- 2750 (C-H). 1H-NMR (CDCl3): δ, 7.65 (d, 1H, 1H-indazole H4 , J= 7.6), 7.35 (d, 1H, indole H7 , J = 8), ,6.80-6.85 (m, 3H, indole H2, H5, H6), 7.35-7.45(m,5H,phenyl group),3.80 (s, 2H, C-CH2 -N), 3.25 (t, 4H, piperazine H3, H5 , J = 4.8), 2.70(t, 4H, piperazine H2, H6 , J = 4.8),Anal. Calc. for: C, 78.32; H, 7.26; N, 14.42%, found: C, 78.18; H, 6.94; N, 14.25%.
Yield: 58%: mp 195.0 ◦C. IR (KBr) cm-1: ν 3100 (N-H), 3020- 2720 (C-H). 1H-NMR (CDCl3): δ, 7.60 (d, 1H, 1H-indazole H4 , J = 7.6), 7.30 (d, 1H, indole H7 , J = 8), 7.40-7.55(m,4H,phenyl group) ,6.60-6.65 (m, 3H, 1H-indazole H2, H5, H6), 3.60 (s, 2H, C-CH2 -N), 3.75 (t, 4H, piperazine H3, H5 , J = 4.8), 2.50(t, 4H, piperazine H2, H6 , J = 4.8), 2.43(s,3H,-CH3 ),2.40(s,3H,phenyl attached CH3 group) , Anal. Calc. for C, 70.32; H, 7.15; N, 14.20%, found: C, 70.18; H, 6.94; N, 14.10%
Yield: 53%: mp 160.0 ◦C. IR (KBr) cm-1: ν 3020 (N-H), 3090- 2710 (C-H). 1H-NMR (CDCl3): δ, 7.10 (d, 1H, 1H-indazole H4 , J = 7.6), 7.20 (d, 1H, 1H-indazole H7 , J = 8), 7.15-7.40(m,4H,phenyl group),6.20-6.15 (m, 3H, 1H-indazole H2, H5, H6), 3.20(s, 2H, C-CH2 -N), 3.10 (t, 4H, piperazine H3, H5 , J = 4.8), 2.20(t, 4H, piperazine H2, H6 , J = 4.8),, Anal. Calc. for: C, 65.32; H, 6.15; N, 12.20%, found: C, 65.18; H, 6.24; N, 12.10%.
Yield: 51%: mp 165.0 ◦C. IR (KBr) cm-1: ν 3000 (N-H), 3010- 2710 (C-H). 1H-NMR (CDCl3): δ, 7.15 (d, 1H, 1H-indazole H4 , J = 7.6), 7.40 (d, 1H, 1H-indazole H7 , J = 8), 7.05-7.25(m,4H,phenyl group), ,6.10-6.15 (m, 3H, 1H-indazole H2, H5, H6), 3.10(s, 2H, C-CH2 -N), 3.15(t, 4H, piperazine H3, H5 , J = 4.8), 2.20(t, 4H, piperazine H2, H6 , J = 4.8), Anal. Calc. for: C, 65.15; H, 6.15; N, 12.20%, found: C, 65.10; H, 6.04; N, 12.05%.
Yield: 49%: mp 175.0 ◦C. IR (KBr) cm-1: ν 3020 (N-H), 3020- 2750 (C-H). 1H-NMR (CDCl3): δ, 7.20 (d, 1H, 1H-indazole H4 , J = 7.6), 7.00 (d, 1H, 1H-indazole H7 , J = 8), 7.30-7.40(m,4H,phenyl group),6.20-6.25 (m, 3H, 1H-indazole H2, H5, H6), 3.15(s, 2H, C-CH2 -N), 3.20(t, 4H, piperazine H3, H5 , J = 4.8), 2.25(t, 4H, piperazine H2, H6 , J = 4.8), Anal. Calc. for C, 64.15; H, 4.15; N, 8.20%, found: C, 64.10; H, 4.04; N, 8.05%.
The anti bacterial activity of synthesized compounds was studied by the disc diffusion method against the following pathogenic organisms. The gram-positive bacteria screened were staphylococcus aureus NCCS 2079. The gram negative bacteria screened were Escherichia coli NCCS 2065 and pseudomonas aeruginosa NCCS 2200. The synthesized compounds were used at the concentration of250 μglml and 500μglml using DMSO as a solvent the Cefaclor 10μglml disc was used as a standard. (Himedia, Laboratories Ltd, Mumbai).The test results presented in the (Table 1), suggest that 4b,4d,4e exhibit high activity against the teased bacteria, the rest of the compounds were found to be moderate active against the tested microorganisms.
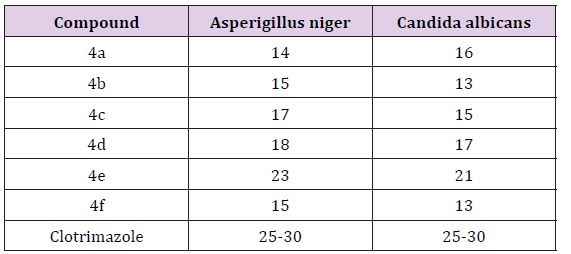
The antifungal activity of synthesized compounds was studied by disc diffusion method against the organisms of Penicillium and Trichophton. Compounds were treated at the concentrations of 500μglm and 1000μglml using DMSO as solvent. The standard used was Clotrimazole 50 μglml against both organisms. The test results were presented in the (Tables 2 & 3).
Table 2: Antibacterial activity by disc diffusion method of indazole linked 1, 3,4 oxadiazole 4(af).
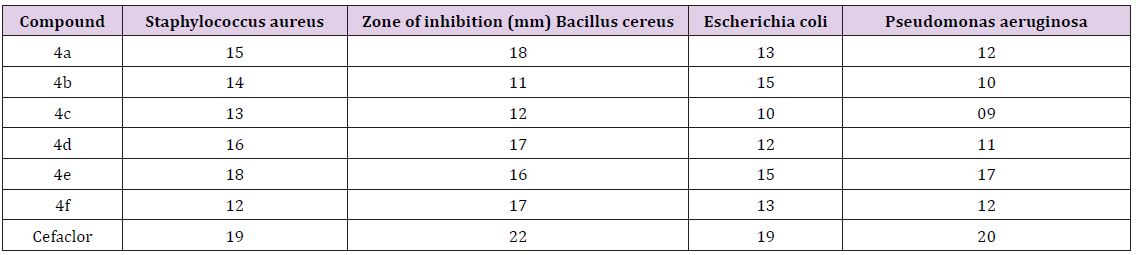
Table 3: Antifungalactivity.
a) It’s my pleasure to express my thanks to Department of Chemistry for giving an opportunity to do research.
b) I express my sincere thanks to P.RAVISANKARA REDDY (Sr. Excutive in Biological E Ltd company, shameerpet, Hyderabad), who is giving valuable guidance during my research.


