Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Vinita Ved1 and Gabriela Fernandes2*
Received: November 11, 2017; Published: November 30, 2017
Corresponding author: Gabriela Fernandes, Department of Oral Biology, SUNY Buffalo, New York, USA
DOI: 10.26717/BJSTR.2017.01.000554
Antimicrobial Peptides (AMPs) are present in the oral cavity in the form of defensins and human cathelicidin LL-37 (from neutrophil granules) and histatins along with pdefensins 1 and 2 (from salivary glands and gingival epithelial cells). The oral micro flora organisms that play an important role in the pathogenesis of periodontal disease are opportunistic pathogens, that are highly proteolytic and this activity is known to contribute to nutrient acquisition, tissue destruction and de-regulation of inflammatory responses. Furthermore, the production of proteases enables oral bacteria to evade killing by antimicrobial peptides, thus contributing to the virulence of such opportunistic pathogens, which could have implications for the use of antimicrobial peptides as therapeutic agents to treat periodontal disease. Hence, this review summarizes the suggestive role of AMPs in periodontal disease.
Key words: Immunity; Anti-Microbial Peptides; Periodontitis
Abbreviations: AMPs: Antimicrobial Peptides, LAP: Lingual Antimicrobial Peptide, HNP: Human Neutrophil Peptide; hBD: Human Beta- Defensins
Humans often require a multilayered defense mechanism to function smoothly and combat micro-organisms and pathogens. The fundamental guarding complex for almost all human organisms comprises of innate immunity [1]. It is this defense mechanism that helps discern a wide variety of agents, known as pathogens, and distinguish them from the organism’s own healthy tissue [1]. The oral cavity is a manifold interaction where sundry organisms, both commensal and destructive types, intercommunicate and escalate in the environment [2]. The noteworthy quality of the oral cavity lies in the presence of specialized interaction between tooth (hard tissue) presenting itself from the underlying gingival epithelium (soft tissue).
Ideally in the oral cavity, the tooth structure often encompasses a layer of pathogenic biofilm termed “Dental plaque,” that compromises the surrounding epithelium via its incessant exposure to microorganisms [3]. This is when the comeback of the epithelium to these insults determines the overall condition of the gingival sulcus. It is evident that the oral epithelium works in several ways for the conservation of the underlying tissues. As a physical barricade, it can counter unbroken microbial oppositions from dental plaque by the production of chemokines, cytokines, and antimicrobial peptides (AMPs), which enhance inflammation and immune response in oral epithelial tissues [4]. These epithelial antimicrobial peptides are considered to be paramount for the innate immunity of the host [5].
AMPs are also prime contributors that enable the stabilization between health and disease in this complex ecosystem [6]. Exhibiting a wide spectrum of antimicrobial activity against grampositive and gram-negative bacteria, yeasts and certain viruses, they possess the ability to prevent various oral periodontal diseases including bacteria, fungal and viral infections [7]. The reaction involving multiple AMPs to a single pathogen of infection prevents the consequences of antimicrobial resistance. Several families of antimicrobial peptides have been studied in the oral cavity which includes α-defensins, β-defensins, calprotectin, adrenomedullin, histatins, and cathelicidin [8]. The aim of this review paper is to view the crucial role of these molecules against periodontal diseases and its function in host immunity. The article also sheds light on the mechanism of action and the types of identified AMPs.
The AMPs react to the periodontopathogenic bacteria in a synergistic manner, whereby they secrete chemical innate immunity signal molecules like interleukin, chemokine and cytokines that attract neutrophils at the site and caution the host response [7]. Conducive with the amount of microbial exposure, they also produce natural AMPs and proteins. By acting as an integral part of the hosts natural innate environment, the oral epithelium, polymorph nuclear leukocytes (neutrophils) and saliva, all concurrently and solitarily bestow to this response [9,10]. These responses involve several salivary antimicrobial peptides, the β-defensins manifested in the epithelium, the α-defensins expressed in neutrophils, and the cathelicidin, LL-37, in both epithelium and neutrophils [11-13].
The first AMPs identified in the oral epithelium were defensin, Lingual Antimicrobial Peptide (LAP) [14]. The defensin families are prominent AMPs that reside in the host environment, which include the alpha-defensins (human neutrophil peptide; HNP) and human beta-defensins (hBD) [15]. Defensins are stored in secretory granules, with expanding levels observed during periodontitis (Figure 1). Their mechanism of action involves destroying phagocytosed bacteria upon fusion with the secretory granules from the phagocytic vacuoles they reside in [16]. Being a part of the host’s natural environment, expressed in gingiva, tongue, salivary glands and mucosa, they are present in conditions like oral inflammation, carcinomas, etc. Defensins have also shown to inhibit LPS-stimulated inflammatory responses in host cells, which is a primary causal mechanism in the pathogenesis of periodontal disease [16]. Defensins are allocated into subfamilies of α- and β-defensins. Human beta-defensins (hBD) are elementally revealed in epithelial cells, while alpha-defensin are predominantly expressed in neutrophils [17]. Segregated in terms of their cysteine motifs, dually both of them share a homogenous secondary structure, and are opulent in cationic residues [18,19].
Figure 1: Mode of activation of hNPs in periodontal tissues.
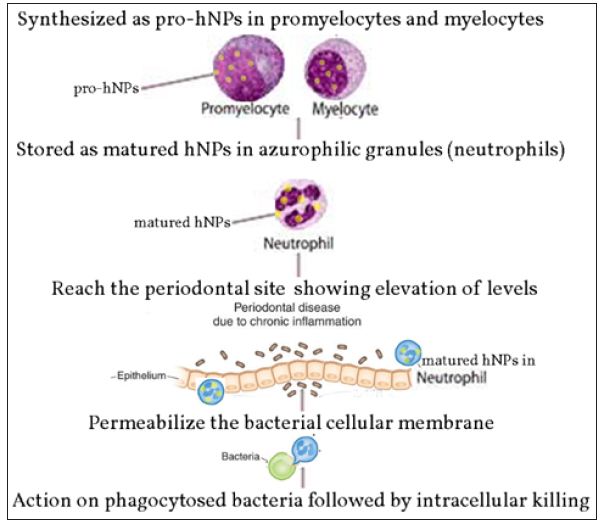
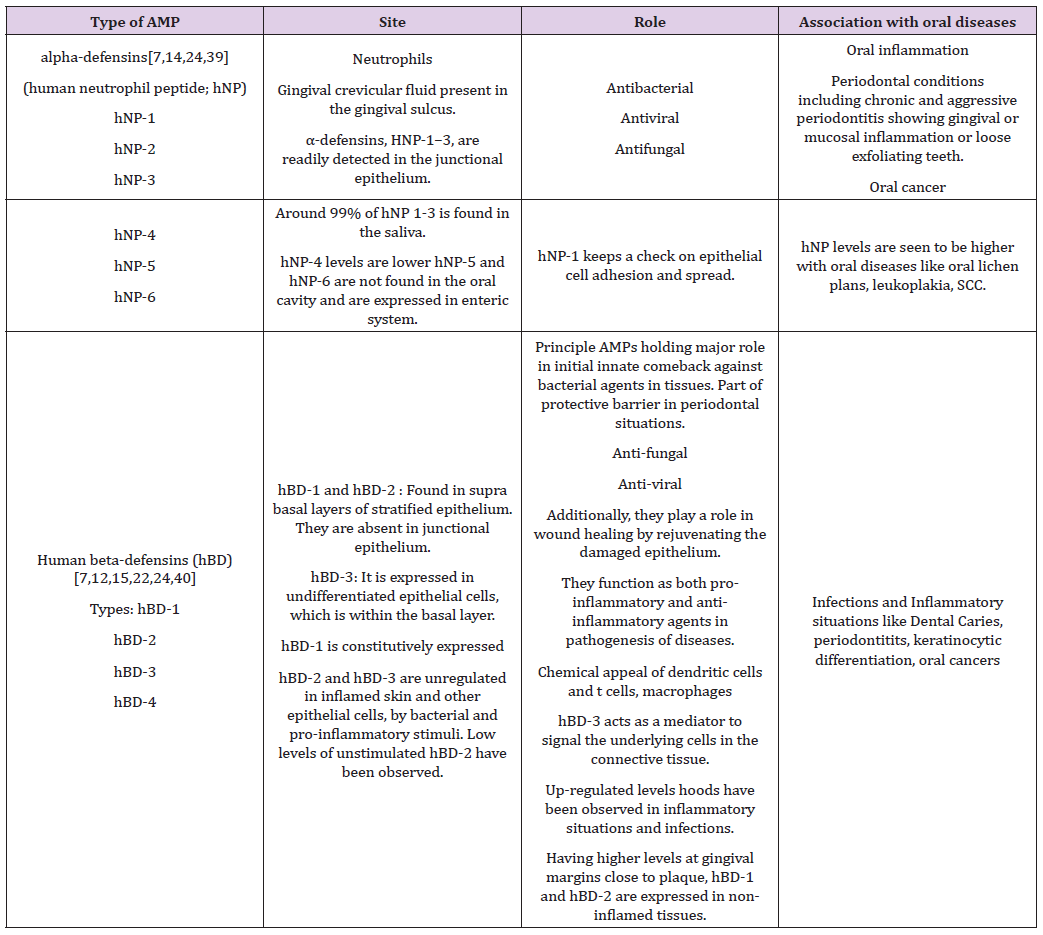
In the α-defensin subfamily, four of the six α-defensins, human neutrophil peptide -1, -2, -3, and -4, are fabricated and gathered in neutrophil granules while the other two α-defensins, HD-5 and -6, are synthesized and stored in the granules of paneth cells, specialized epithelial cells at the crypts of Lieberkuhn of the small intestine [17,20,21]. In the β-defensin subfamily only hBD -1, -2 and -3 are substantially expressed in the oral cavity [16,22]. hBD-3 along with the respective three mentioned above are markedly expressed in epithelial cells that encrust some tissues and organs, fundamentally skin [23]. In non-inflamed gingival tissues and gingival crevicular fluid, hBD-1 and hBD-2 have been expressed, with maximum levels at gingival margin [22]. Elevated levels of HNP1-3 have been noted in cases of periodontal infections, indicating a strong correlation See Table 1 for summary.
Table 1: Summary of the role of defensins.
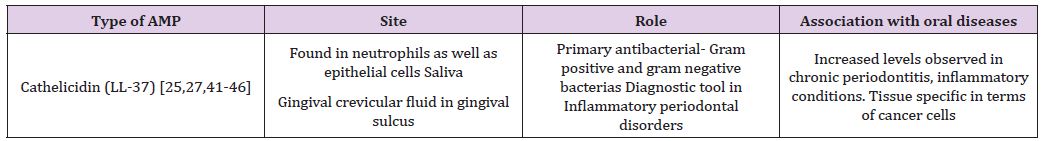
Used markedly as one of the predominant identifying tools in inflammatory periodontal disorders, this class of AMPs incorporates a mature peptide and cathelin domain. It is a multifunctional peptide, comprising of 37 amino acids [24]. Active against gram positive and gram negative bacterias, it directly binds to the LPS of bacterial cells, and is native to the oral cavity in several sites, including the buccal and tongue mucosa, gingival crevicular fluid [24]. By activating antigen-presenting cells, it presents as a hemoattractant for immune cells, including monocytes, T cells, etc. There is a specific correlation amongst multiplication in LL-37 levels and periodontal inflammation [25-28]. It is also known to cause an elevation in mucosal thickness in the gingiva. Furthermore, studies have also demonstrated its tissue specific effects on cancer cells [27,28]. Their mode of action involves intracellular killing of the phagosomes after phagocytosis of the bacteria, where the AMP in the neutrophil is severed into a fully developed peptide (Table 2).
Table 2: Summary of the role of Cathelicidin.
These AMPs are exclusively resided in the salivary glands, i.e. parotid and submandibular salivary duct cells. Histamine 1, 3 and 5 are found to be predominant of the total histatin proteins (85%) in the saliva [29-32]. They have a major role in fungicidal activity, having a noteworthy role in oral candidiasis restraint, especially histatin-5, which is the most significantly active against candida species as well as bactericidal activities against Porphyromonas gingivalis and Streptococcus mutans, which play a key role in the etiopathogenesis of periodontal disease and dental caries ,respectively [30,32]. Significant linkage occurs between xerostomia and oral candidiasis. It is also noteworthy that the antifungal action is shared between alpha-defensin and histatin, which require ATP transport found in active mitochondria.
Another AMP identified from the parotid salivary gland is SLPI. Manifested in neutrophils, epithelial cells and salivary glands, this protein suggests anti-inflammatory action at the site of infection [33]. Supplementary to having fungicidal action, it has shown to inhibit contamination of human monocytes and is also conveyed in oral tumor tissues as well as inhibition of the HIV virus [34,35]. SLPI is associated with wound repair and its expression is elevated following wound healing [35,36]. The elevated quantity of SLPI following periodontal treatment is an evidence of inflammation resolution activities [36].
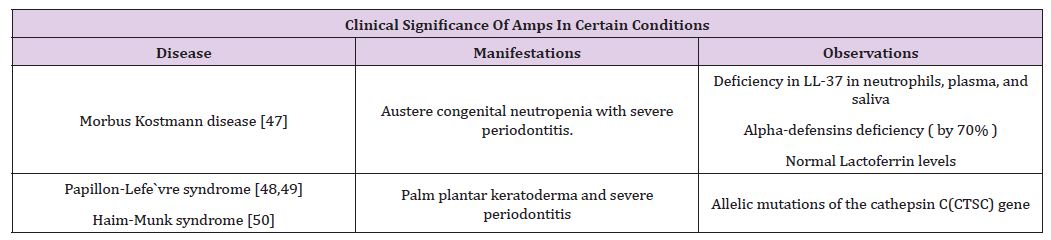
The capacity of these inborn antibiotics is only at its inception of its recognition, as amplified natural announcement or as novel relieving agents [8,37]. This class of proteins is a captivating target for periodontal diseases [37]. In times of periodontal disease, AMPs amalgamate with other inflammatory proteins and maintain inflammatory molecules and pathways. It can be concluded that salivary AMPs have a prospective capacity to be recognized as initial markers of periodontal infections [38]. Thus, a dual approach is mandatory to comprehend the task of hosts’ immunity in response to, against periodontitis [37]. This involves more comprehensive statistics about HNP and hBD, LL-37, and other oral antimicrobial peptides and proteins, along with their mode of action and clinical significance [38-50]. These peptides are peculiar in keeping the level of bacteria in control, having distinctive as well as dual roles in maintaining oral health. Not only can they act as diagnostic or prognostic biomarkers, but also the enhancement of new peptide agents can be a signaling method for future investigation and testing (Table 3).
Table 3: Significance of AMPs.
Moreover, Antimicrobial peptides are potentially important as novel therapeutic agents against periodontal disease diagnosis and potential treatment and probably show most immediate promise for development as topical adjuvant agents in conjunction with conventional periodontal therapy in the treatment of periodontal disease. This makes them promising for oral diseases, as topical application of antimicrobial agents is easy and appropriate, and their use would not contribute to resistance to antibiotics normally used in treatment of more life-threatening bacterial infections. All these findings will have direct implications for new understanding of oral innate immune responses and the development of potential new and innovative therapeutic interventions for periodontal disease.


