Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Manisha Bansal*, Kulvir Kaur, Jyoti Tomar and Lakhvir Kaur
Received: November 09, 2017; Published: November 14, 2017
Corresponding author: Manisha Bansal, Department of Chemsirty, Punjabi University, Patiala-147002, India
DOI: 10.26717/BJSTR.2017.01.000530
Medicinal properties of the plants are attributed due to presence of various flavonoids. Flavone is one class of flavonoids which attracted the attention of researchers worldwide. These ‘yellow’ colored compounds have 2-phenylchromen-4-one backbone structure. Flavones contribute to various medicinal properties like anti diabetic [1], anti-inflammatory [2], anti-oxidant [3] and anti-cancer [4]. In some basic methods of synthesis of flavones aldehydes react with hydroxyl aceto phenones under claisensmith condensation. In this review we have tried to summarize the various methods of synthesis of flavones.
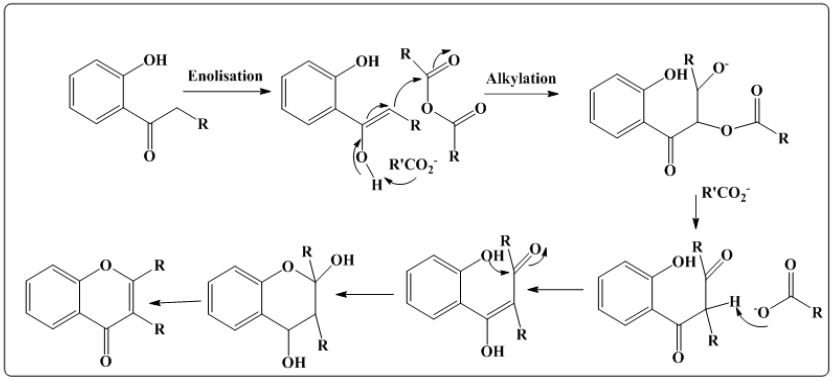
Allan J and Robinson R [5] carried out the reaction of o-hydroxyaryl ketones with aromatic anhydrides which results in the formation of flavones and isoflavones shown in (Figure 1).
Figure 1: Synthesis of flavoves by Allan Robinson reaction. O-benzoyloxyacetophenone is heated with glycerol at 2600 C for two hrs which leads to the formation of flvone.
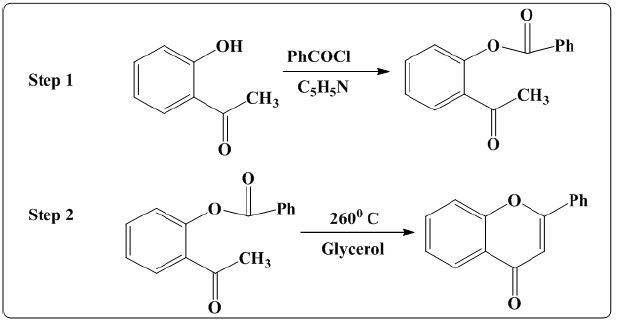
i. Method 1: T S Wheeler [6] carried out synthesis of flavones via two step synthesis method. In first step O-benzoyloxy aceto phenone was synthesized from reaction of O-hydroxy aceto phenone with benzoyl chloride in the presence of pyridine. In the second step O-benzoyloxy aceto phenone is heated with glycerol at 2600 C for two hrs which leads to the formation of flvone. The reaction is shown as (Figure 2).
Figure 2: Synthesis of flavones using O-benzoyloxyacetophenone.
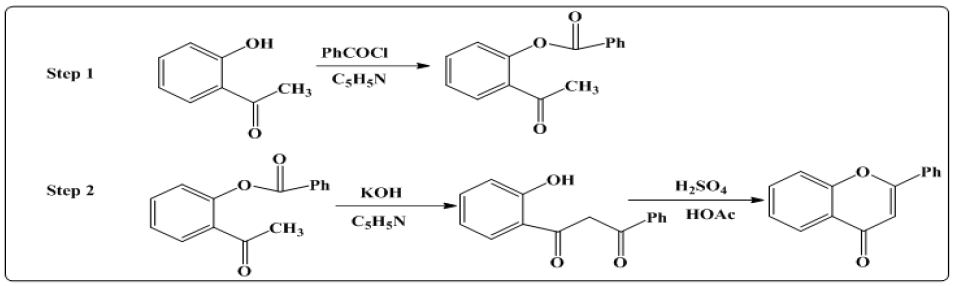
ii. Method 2: In the second method7 first step is same as shown in method 1. Then in the second step O-benzoyloxyacetophenone was converted into di ketone using KOH and pyridine. Then diketone is refluxed with glacial acetic acid and sulphuric acid for 1 hr to obtain the final product as shown in (Figure 3).
Figure 3: Synthesis of flavones using diketone.
In this method [8] O-chloro phenyl benzoyl acetylene in the presence of morpholine was refluxed for 10 hrs which ends up with the flavone as final product via removal of morpholine hydrochloride. The reaction mechanism is shown as (Figures 4 & 5).
Figure 4: Synthesis of flavones involving cyclization via displacement of aromatic chlorine.
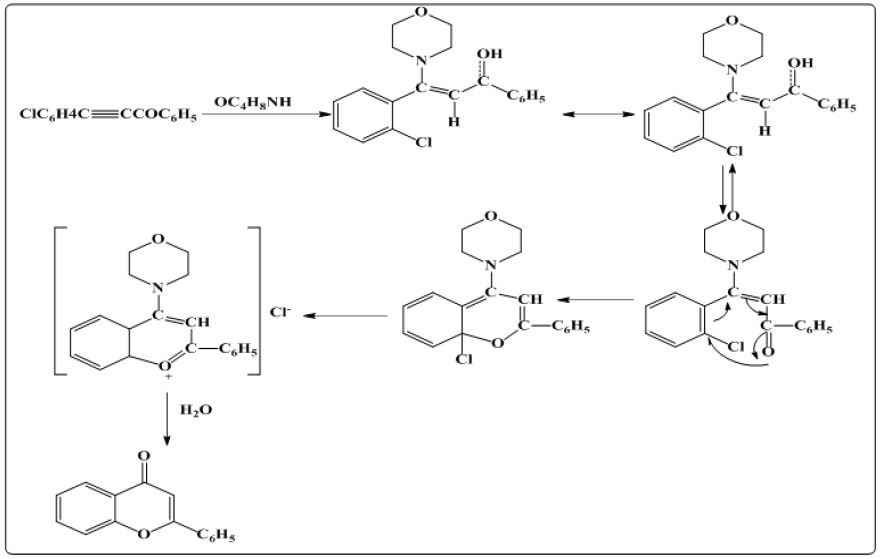
Figure 5: Synthesis of flavones using ium chloropalladite or palladium (II) acetate catalysed reaction.
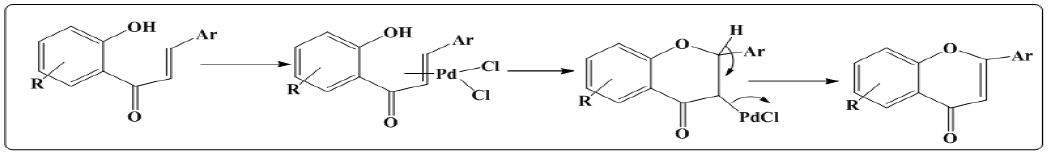
i. Lithium chloropalladite or palladium (II) acetate catalysed reaction: Initially benzene was stirred with sodium methoxide and then refluxed with Lithium chloropalladite or palladium (II) acetate and at the end reaction results in the formation of flavones [9].
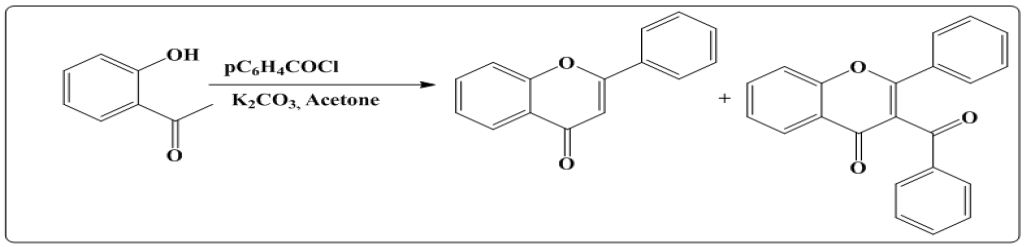
ii. Using LiHDMs and acid catalysed cyclization reaction of O –hydroxyacetophenone: Reaction of O-hydroxyacetophenone with benzoyl chloride under acidic condition using LiHDMs leads to the formation of flavones in addition to the side product [10]. Reaction is shown in (Figure 6).
Figure 6: Synthesis of flavones using LiHDMs acid catalysed cyclization reaction of O –hydroxyacetophenone.
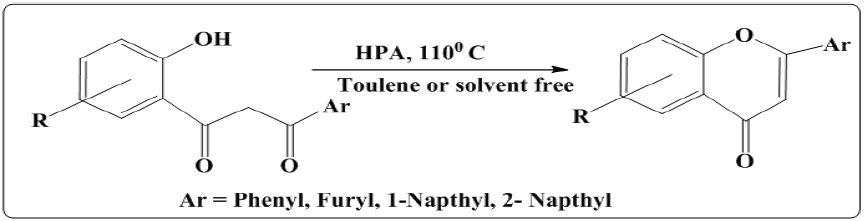
iii. Wells-Dawson method of synthesis of flavones using Heteropoly acid (HPA): In this method [11] synthesis was carried out using substituted 1,3 diketones dissolved in toluene or solvent free environment. Recation mixture was refluxed for 5hrs. at room temperature without catalyst (WD40/SiO2) no product was identified on TLC plate whereas use of just 1% mmol of HPA push the reaction in forward direction as shown in (Figure 7).
Figure 7: Wells-Dawson method of synthesis of flavones.
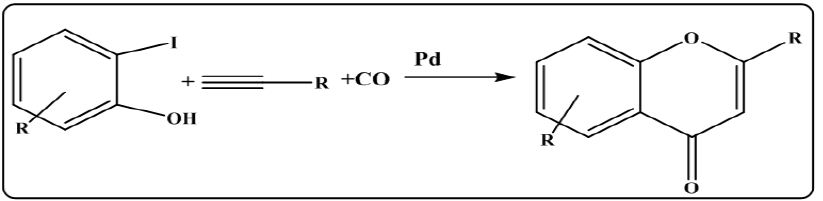
iv. From idophenol with alkynes in the presence of pladinum catalyst: Reaction of substituted idophenols with alkynes in the presence of carbon monoxide and pladinum catalyst yielded the flavones [12] as shown in (Figure 8).
Figure 8: Synthesis of flavones from idophenols and alkynes.
i. Iodine-alminium trioxide (I2/Al2O3) microwave assisted synthesis of flavones: In this method substituted O-hydroxyacetophenones was irradiated in microwave with carboxaldehyde in the presence of I2-Al2O3/NaOH [13]. Reaction mechanism is shown in (Figure 8).
ii. From the replacement of alkene hydrogen of 4H-chromen- 4-one: Reduction of 4H-chromen-4-one in presence of 2,2,6,6-Tetramethylpiperidine (TMP),Zn/ MgCl and THF gives the flavones as final product [14].
In this review we attempted to present various methodologies for synthesis of flavones. This review will help to researchers in this field to find the efficient method.


