Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Pritam S Jain*, Harshal P Chaudhari, Gayatri B Patil and Sanjay J Surana
Received: October 13, 2017; Published: October 24, 2017
Corresponding author: Pritam S Jain, RC Patel Institute of Pharmaceutical Education and Research, Karwand Naka, Shirpur, Dist Dhule 425 405 (MS)
DOI: 10.26717/BJSTR.2017.01.000461
The objective of the work was to study the degradation behavior of rizatriptan benzoate under different ICH recommended stress conditions by HPLC, and to establish a validated stability indicating LC assay method. Rizatriptan benzoate was subjected to stress conditions of hydrolysis and oxidation decomposition. Extensive degradation was found to occur in acidic medium. Mild degradation was observed in alkaline and oxidative conditions. Rizatriptan benzoate was stable to photolytic and thermal stress conditions. Successful separation of drug from degradation products formed under stress conditions was achieved on a Perfectsil (C18, 250 mm × 4.6 mm, 5.0 μ) and 0.01 M Phosphate buffer : methanol (80:20 v/v) as the mobile phase at a flow rate of 1.0 mL /min at ambient temperature and detected at 225 nm. pH of buffer is adjusted 5.0 with 85 % of otho phosphoric acid. Characterization of the degradent product was separately. The molecular weight of impurity product was found to be 188.
Keywords : Rizatriptan Benzoate; Rp-Hplc, Validation, Purity Evaluation; Degradation Product
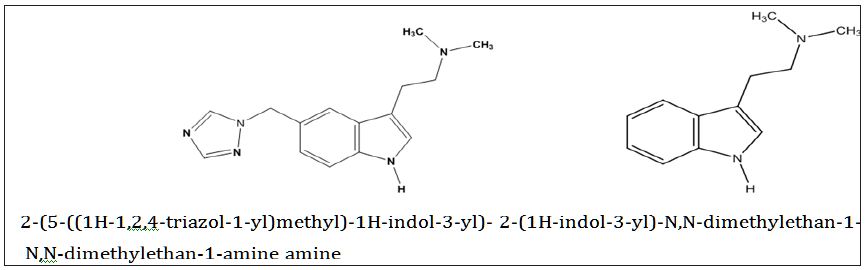
Rizatriptan benzoate (Figure 1) is a triptan drug used for the treatment of migraine headaches. It is a selective 5-Hydroxytryptamine 1 receptor subtype agonist [1]. Since there are only few methods are available for the determination of Rizatriptan benzoate. The present work is an attempt to estimate the same by a new force degradation method. The literature review shows very few methods for the determination of Rizatriptan benzoate and pharmaceutical validations by HPLC method but that various other methods like UV spectroscopic method for Rizatriptan benzoate [2,3], HPLC method for Rizatriptan benzoate [4], LC-MS/MS method for determination of Rizatriptan benzoate in human plasma [4]. This method can be successfully used for routine analysis of Rizatriptan benzoate as it is rapid, simple, selective and sensitive method for the determination using High Performance Liquid Chromatographic (HPLC) technique [5].
Figure 1 : The chemical Structure of Rizatriptan Benzoate and its degradation impurity.
Impurity profile of an active pharmaceutical ingredients (APIs) and evaluation of their toxicity effect is necessary step in developing a safe and effective drug and is essential for medical safety reasons [6]. It is mandatory that, any new impurities present in the drug substance and drug product above the threshold limit need to be identified and characterized. The present manuscript describes the Stability-Indicating RP-HPLC Method and identification and characterization of acidic degradation impurity of RizatriptanBenzoate Drug Substance as well as Drug Product [7-9]. Therefore, the objective of the reported research was to study degradation of rizatriptan benzoate under different International Conference of Harmonization (ICH) recommended stress conditions and to evaluate the degraded products by MS and to establish a stability-indicating RP-HPLC method for accurate quantification of rizatriptan benzoate in pharmaceutical dosage forms [10].
Pure samples of Rizatriptan Benzoate were obtained from Cipla Pharmaceutical Limited as a gift sample. HPLC grade Methanol, AR grade Triethylamine and Orthophosphoric acid was used. Highly pure water was prepared by double distillation and filter through with 0.45μ membrane filter. Hydrochloric acid, Sodium hydroxide and Hydrogen peroxide were used of laboratory grade.
Agilent HPLC system equipped with low pressure quaternary gradient pump along with photo diode array detector and manual rheodyne sample injector has been used for the analysis of samples. The data was collected and processed using Ezichrom Elite software. A LC GC RP-18.5μm. (250x4.5mm) BDS column was employed for the separation of impurity from Rizatriptan Benzoate. The column eluent was monitored at 225nm. The sample diluents was a mixture of 7 ml Triethylamine in 1000 ml water of pH 5.0 adjusted with orthophosphoric acid and methanol in the ratio of 8:2 (v/v), filter through 0.45μ or finer porosity membrane filter.
A simple isocratic reverse-phase HPLC method was optimized for the separation of degradation product where the mobile phase A and B are 7 ml Triethylamine in 1000 ml water (pH adjusted to 5.0 with orthophosphoric acid) / methanol, respectively. HPLC method for drug substance: The solvent composition was held at 80 % mobile phase A and 20% mobile phase B. The Flow rate was 1.0 ml/min. The volume injected with Rheodyne manual sampler injector with 20 μL capacity. The chromatographic run time was 15 min. HPLC method for drug product (tablet) was same.
An Agilent preparative HPLC system equipped with liquid controller pump, photodiode array detector, and manual sample injector fitted with 20 μL loop was used. The data was collected and processed using Ezichrom Elite software. An LC GC BDS C18 column (250×4.5mm, 5-Micron) was employed for loading the sample. An analytical method was developed in isocratic mode separately to resolve this degradation product, followed by scaling up the same method for prep-HPLC to collect the required impurity fractions. The mobile phase consists of same composition as described above section. The solvent composition is same as described earlier. The flow rate was set at 1.0mL/min. Detection was carried out at 225 nm. Approximately 100 μg/mL of sample was prepared using a sample diluent. The sample diluent was a mixture of mobile phase in ratio 8:2.
Initial LC/MS analysis has been performed on Varian Inc (USA) 410 Prostar Binary LC with 500 MS IT PDA Detectors. The analysis was performed in positive ionization mode with turbo ion spray interface. The parameters for ion source voltage IS=5500V, declustering potential, DP=70V, focusing potential, FP=400V, entrance potential, EP=10V were set with nebulizer gas as air at a pressure of 40 psi and curtain gases nitrogen at a pressure of 25 psi in mass spectrometer. Further to get accurate mass, analysis was performed on high resolution mass spectrometer using electrospray ionization. The accurate mass obtained from the instrument, theoretical mass and mass error was calculated.
The 1H experiment was carried out for unknown impurity at processional frequencies 400.1328 MHz at 25° Cona Bruker Avance- 300FT NMR spectrometer. The 1H chemical shift is recorded on the δ scale in ppm, relative to tetramethylsilane (TMS) δ 0.00 in ppm.
The mobile phase-A containing of 7ml Triethylamine in 1000 ml buffer pH 5.0 and mobile phase-B consist of Methanol flow in ratio 80:20. Where a column BDS C18 (250mm × 4.5 mm, 5 micron) was found to resolve Rizatriptan benzoate. The mobile phase was filtered through 0.45 μ membrane filter and the sonicated for 10 min. The flow rate was set at 1.0 ml/min. The drug showed good absorbance at 225 nm, which was selected as wavelength for further analysis all determinations were performed at ambient column temperature. Sample Diluents was used mobile phase-A and mobile phase-B in the ratio of 80:20 v/v.
Accurately weighed 20mg of rizatriptan benzoate, dissolved in 50 ml of volumetric flask with diluent (Stock solution), respectively. The stock solution was further diluted by using mobile phase to get the concentration of 100 μg/ml of rizatriptan benzoate.
Preparation of the degradation products : The different stress conditions were used for the forced degradation studies of bulk drug and drug formulations. In this procedure make one sample without drug i.e. placebo sample and sample with drugs were compared with force degradation sample. The stress sample was detected at 225 nm wavelength and run time was taken as same as assay sample.
Acidic Condition : For Acid hydrolysis, 2N of HCl was used for preparation of 100 μg/ml RZT solution. RZT API taken 50 mg was dissolved in 50 ml of volumetric flask with 10 ml mobile phase, respectively and makes sample preparation for tablet equivalent to 20 mg of RZT in 100 ml volumetric flask. Then add 5 ml of 2N HCl in flask and exposed 90o at 8 hrs. After it add 5 ml of 1N NaOH in flask for neutralization of reaction. Then make up with mobile phase. For further dilution take 5 ml of each sample in 50 ml of volumetric flask individually and for tablet degradation, 5 ml taken in 20 ml of flask and make up with mobile phase.
Alkaline Condition: For Base degradation study, 2N NaOH was used. 50 mg of rizatriptan benzoate was taken in 50 ml volumetric flask containing 10 ml of mobile phase. The sample preparation for tablet equivalent to 20 mg of RZT in 100 ml volumetric flask was taken containing 10 ml of mobile phase. Then in both the stock solution add 10 ml of 2N NaOH and exposed 90o at 8 hrs. To neutralize the solutions add 1N HCl in each flask. Make up volume up to mark with mobile phase. For further dilution take 5 ml of sample stock solution in 50 ml of volumetric flask and take 5 ml of tablet degradation stock in 20 ml of flask and make up the volume with mobile phase to achieve the concentration of 100 μg/ml.
Oxidation Condition: For Peroxide degradation, 3% H2O2 was used. Rizatriptan benzoate API taken 50 mg was dissolved in 50 of volumetric flask with 10 ml mobile phase, respectively and makes sample preparation for tablet equivalent to 20 mg of RZT in 100 ml volumetric flask. Then add 5 ml of 3% Hydrogen peroxide in each flask and exposed to 1 hrs at room temperature. Then make up with mobile phase. For further dilution take 5 ml of each sample in 50 ml of volumetric flask individually and for tablet degradation, 5 ml taken in 20 ml of flask and make up with mobile phase.
Detection of Impurities by HPLC: Typical HPLC chromatogram of Rizatriptan Benzoate and its degradation product observed in drug substance as well as in drug product obtained by using the HPLC method.
Isolation of 2-(1H-indol-3-yl)-N,N-dimethylethan-1-amine Impurity by Prep HPLC : A simple reverse phase chromatographic system, discussed under experimental section was used for isolating the unknown degradation product 2-(1H-indol-3-yl)- N,N-dimethylethan-1-amine. In this chromatographic system, the 2-(1H-indol-3-yl)-N,N-dimethylethan-1-amine Impurity eluted at about 9.48 min. So fractions eluting between 2.7 and 10 min. and sample was sent for characterization by NMR, Mass experiments.
Mobile phase consisting of different buffers with methanol at different buffer-methanol ratio and at different mobile phase pH was tried but peak shape and retention time of Rizatriptna benzoate was found to be broad compared to buffer-acetonitrile composition as mobile phase. After various trials of different buffer and acetonitrile ratio as mobile phase, Potassium dihydrogen phosphate was selected as buffer, pH was adjusted to 5.0 with orthophosphoric acid and buffer-methanol ratio was selected as 80:20 proportions. It showed good resolution of chromatogram with symmetrical peak. The proposed chromatographic conditions were found to be appropriate for the quantitative determination. System suitability tests were carried as per ICH guidelines and parameters are summarized in (Table 1).
Table 1 : Rizatriptan Benzoate exposed to different degradative pathways.
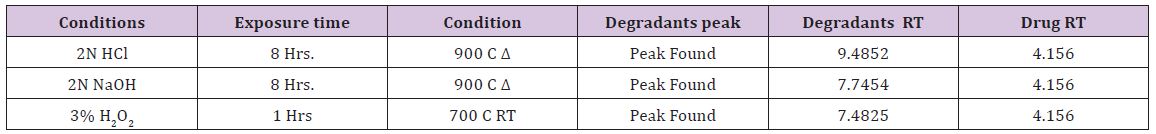
a) The chromatographic conditions are remained same for degradation study
Degradation was not observed for rizatriptan benzoate samples during stress conditions like heat, UV and light, except in base, acid and oxidation. Chromatogram of rizatriptan benzoate standard solution shown in (Figure 2A). Rizatriptan benzoate was degraded into acid (Figure 2B), and base (Figure 2C) and forms polar impurities. In the acidic condition 48.82%, in the basic condition 13.86% and in the oxidative condition 9.27% Recovery was observed for Rizatriptan benzoate (Figure 2D). Peak purity results indicate that the Rizatriptan benzoate peak is homogeneous in all stress conditions tested. Results of rizatriptan benzoate exposed to different degradative pathways shown in (Tables 1-3).
Table 2 : System suitability study of Rizatriptan Benzoate and degradation product (impurity).
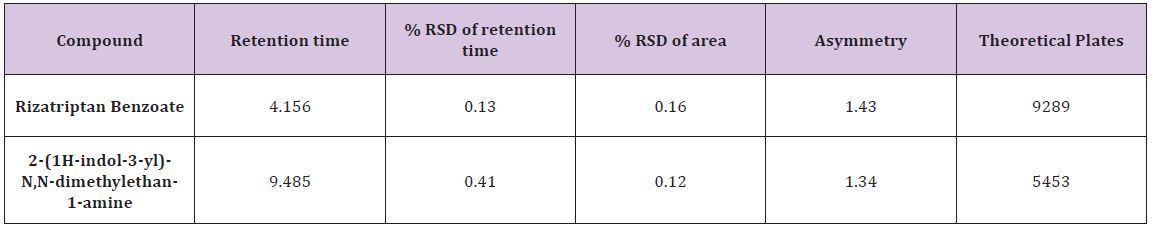
Table 3 : 1H NMR assignments for Rizatriptan Benzoate and 2-(1H-indol-3-yl)-N,N-dimethylethan-1-amine impurity.
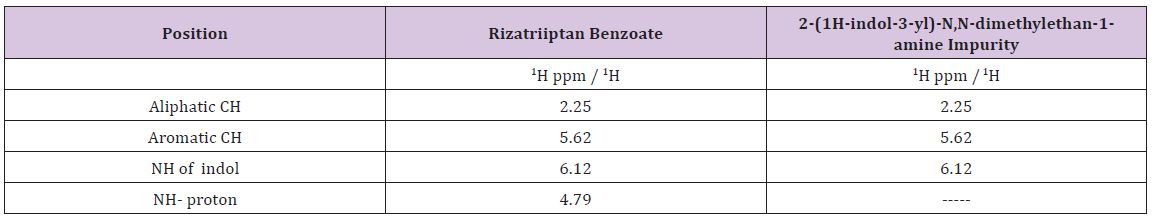
Figure 2A : Chromatograms of RZT standard solution (100 μg/mL).
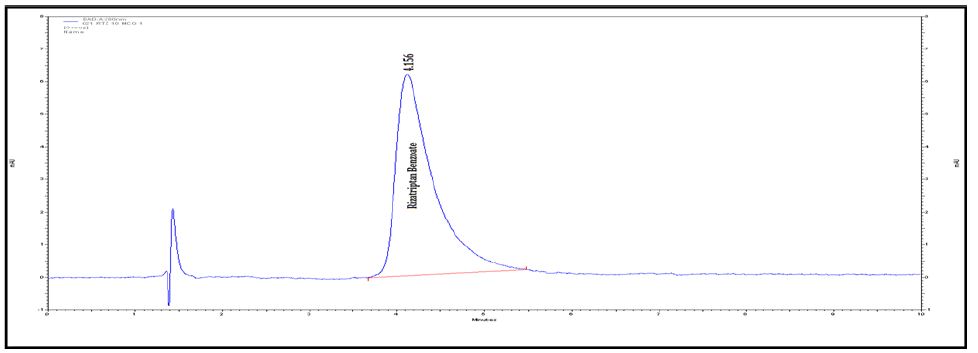
Figure 2B : Chromatograms of RZT standard solution (100 μg/mL).
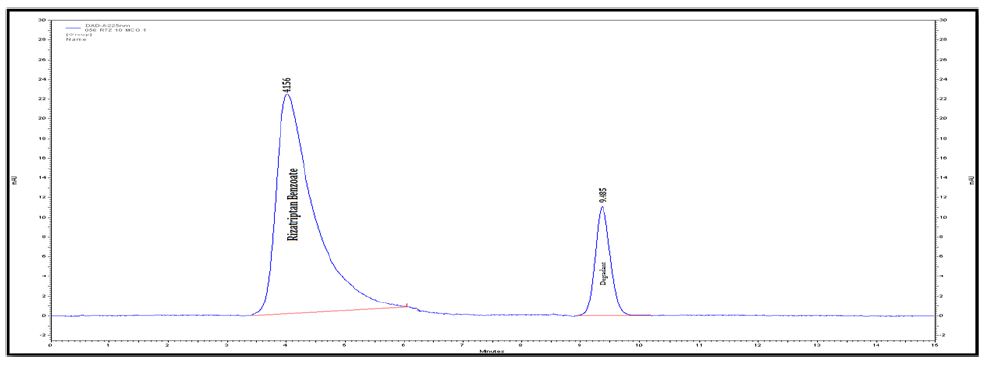
Figure 2C : Chromatograms of RZT standard solution (100 μg/mL).
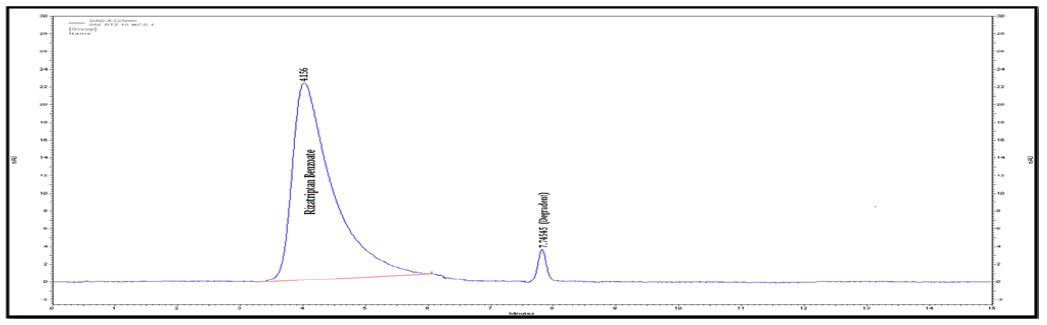
Figure 2D : Chromatograms of RZT standard solution (100 μg/mL).
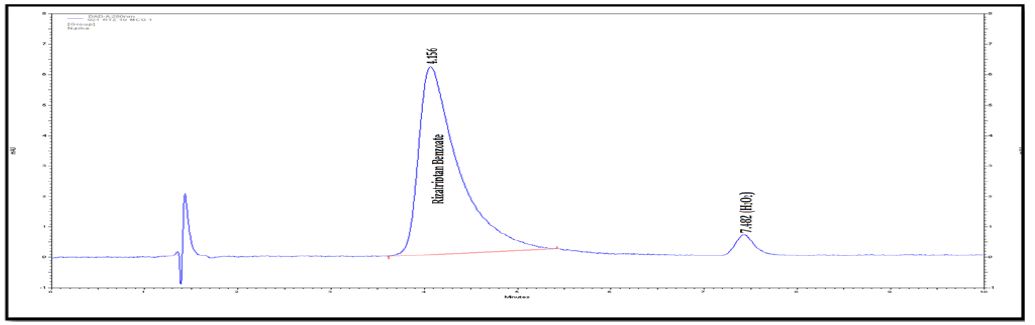
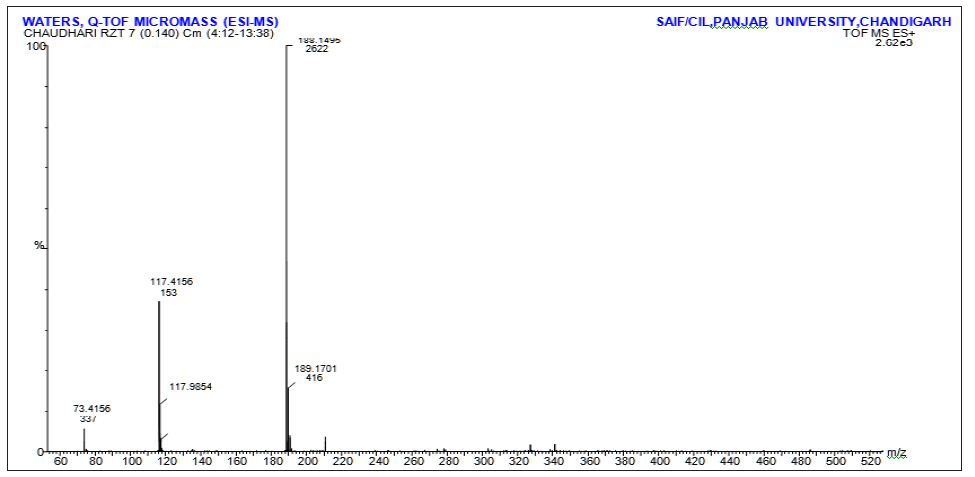
b). Identification of Acidic degraded product of Rizatriptan benzoate: Rizatriptan benzoate drug substance and rizatriptan benzoate tablets were subjected to stability as per ICH guidelines. The mobile phase composition is remained same for the LC-MS. The retention time is 3.30 min for degradation product. The LC-MS analysis showed the m/z value for this unknown impurity as 188 [M+H] + in HPLC method. To further investigate the chemical structure of the unknown impurity, Rizatriptan benzoate drug substance sample was kept at 90°C for 1 Hrs. This sample was subjected to LCMS/ESIQ-TOF. The high resolution mass analysis using Mass Lynx fragmentation tool, proposed the following probable elemental compositions/ molecular formula: C15H19N5.
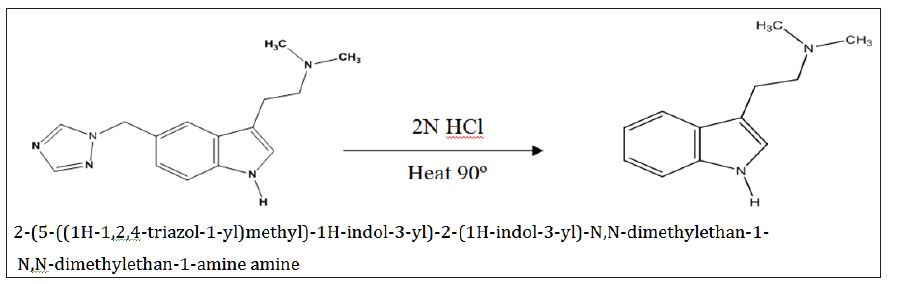
Based on the high resolution mass fragmentation study in comparison to the reported fragmentation pattern of Rizatriptan benzoate, the chemical structure of the unknown impurity of m/z 188, assigned as 2-(1H-indol-3-yl)-N,N-dimethylethan-1-amine impurity. The observed LC-MS Q-TOF fragments of Rizatriptan benzoate acidic degradation impurity m/z 188 is shown in Table 4. Subsequently, 1H NMR spectra of the isolated compound of unknown impurity 2-(1H-indol-3-yl)-N,N-dimethylethan-1-amine compared with that of rizatriptan benzoate was described in Table 5. Based on the above high resolution mass spectral data and NMR data, it is proposed that the unknown impurity is 2-(1H-indol-3-yl)- N,N-dimethylethan-1-amine (Figure 1). The possible mechanism for the formation this impurity is shown in (Figure 3). Rizatriptan benzoate was found to be susceptible to acidic stress (in solution form).
Table 4 : Mass fragmentation of Rizatriptan Benzoate and the impurity mass m/z 189.0.
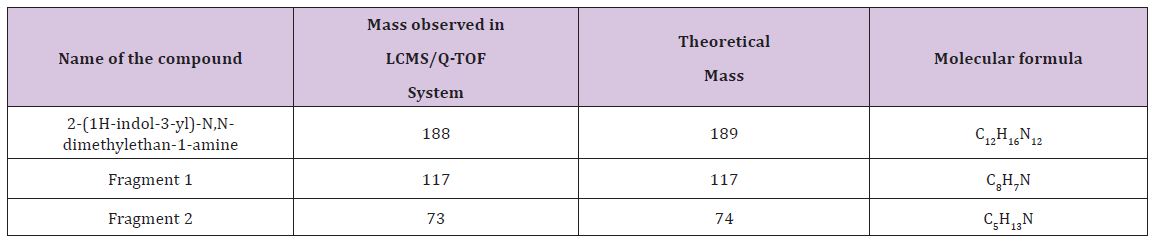
Figure 3 : The degradation pathway of the impurity.
The summary of results from forced degradation studies of RZT and the percentage of drugs remained after undergoing stress. The chemical structure of Rizatriptan benzoate was studied and shown that there are one preferred sites of acidic degradation is formation of degraded product. The degradant was found to be 2-(1H-indol- 3-yl)-N,N-dimethylethan-1-amine impurity. The MS/ESI using selected ion monitoring in the positive ion mode provided a highly selective method for the determination and characterization of rizatriptan benzoate and its degradation product, respectively. The results of NMR and LC-MS are summarized in (Tables 2 & 3) and NMR, LC-MS Spectrum shown in (Figures 4 & 5).
Figure 4 : The degradation pathway of the impurity.
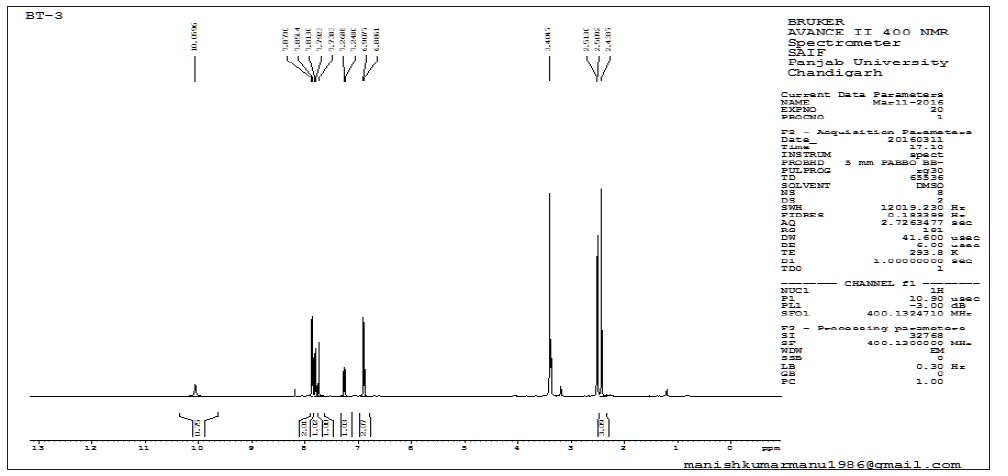
Figure 5 : The degradation pathway of the impurity.
As out lived by ICH guidelines, identification, isolation of impurity is very important task during drug synthesis and storage. It can provide crucial toxicology and safety data of finished drug and dosage forms. We have identified one impurity in aged and stressed samples of Rizatriptan drug substance and drug product. This is characterized by analytical data. The results indicate that the one impurity is from degradation of Rizatriptan benzoate. Formation of this degradation product in stressed.
The authors are thankful to department of SAIF, Punjab University, Chandigarh, for providing us the facility of LC-MS and NMR. The authors are mostly thankful to Cipla research center for providing valuable drugs.


