Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Alcaraz Rubio Jesús*
Received: August 27, 2017; Published: September 11, 2017
Corresponding author: Alcaraz Rubio Jesús, Coordinator of the unit of Hematology in Unión Murciana de Hospitales, Murcia, Spain and the Regenerative therapy unit of Hospital la Milagrosa, Madrid, Spain
DOI: 10.26717/BJSTR.2017.01.000345
Introduction: The diversity of procedures for obtaining platelet and plasmatic growth factors, the absence of control in most of them and the growing field of clinical application, makes them necessary methods adequately structured, documented, controlled and tested, playable by any author. The present work aims to introduce and test a specific technique for obtaining PRP, with precise characteristics both production and final composition of compound got, in 350 hematological healthy patients, comparing our results with those obtained by other procedures scientifically tested.
Material and Methods: 350 caucasian patients were selected, 175 male and 175 female with age range between 15 and 65, healthy haematologically. The procedure for obtaining the PRP, consisted of a single centrifugation of the blood sample for 30 minutes at 3500 rpm in a angular shaft of 16 tubes centrifuge serie (CEMCON 2) and micropipetting the protein fraction rich in platelet and plasmatic growth factors and cell through open technique under aseptic conditions in horizontal laminar flow hood Grade A at a temperature of 22°C, with the use of leuco-platelet or Buffy-coat layer (PRP rich in leukocytes).
Results: No correlation between the amount of concentrated platelets and the amount of growth factors finally obtained was observed. The protocol set forth concentrated levels of platelets and leukocytes approximately 3 to 5 times higher than baseline levels with a predominance of mononuclear. Levels of growth factors from 7-10 times greater than the patient’s baseline levels, with little variation in them. The growth factor levels were stable in the blood of each patient within 24 h of treatment between 7 and 9 times higher compared to the previous baseline
Conclusion: Compared with other procedures discussed in the literature; This method achieves concentration between 1.5 and 3 times more platelets in the final product, as we can see in figure 5, with a purification of growth factors overall type VEGF and TGF-B clearly superior, visible in figure 6.
Keywords: Platelet Rich Plasma; Growth Factors; Centrifugation; Buffy-Coat
The use of platelet growth factors or what is commonly known as Platelet-rich plasma (PRP), is becoming a technique considered medication, despite sharing features of the autotransfusion, with multiple clinical applications in different fields of medicine: trauma and sports medicine, dentistry and maxillofacial surgery, plastic surgery and burns, dermatology, Neurology and Neurosurgery etc. The diversity of existing procedures, the absence of control in most of them and the growing field of clinical application, makes them necessary methods adequately structured, documented, controlled and tested, playable by any author in view to carry out monitoring and traceability of the final product, its possible therapeutic effects as well as side effects that could occur. It is the closest thing to the elaboration of a sheet of the concentrate produced in order to guarantee maximum efficiency and safety for the patient [1-3]. The present work aims to introduce and test a specific technique for obtaining PRP, with precise characteristics both production and final composition of compound got, in 15 hematological healthy patients, comparing our results with those obtained by other procedures scientifically tested.
350 caucasian patients were selected, 175 male and 175 female with age range between 15 and 65, healthy haematologically following analytical standards autologous inclusion of the Spanish Society of Hematology and Hemotherapy regarding biochemical hematological and serological before obtaining samples of whole blood controls. A closed system blood by Vacutainer tube connected to 3.5ml EDTA was used. Forearm venous access was used 20-gauge needle for adults and 22 G for children. The procedure for obtaining the PRP, consisted of a single centrifugation of the blood sample for 30 minutes at 3500 rpm in a angular shaft of 16 tubes centrifuge series (CEMCON 2) and micropipetting the protein fraction rich in platelet and plasmatic growth factors and cell through open technique under aseptic conditions in horizontal laminar flow hood Grade A at a temperature of 22°C, with the use of leuco-platelet or Buffy-coat layer (PRP rich in leukocytes). In the final product, we proceeded to cell counting by hemocytometer Coulter (Beckman), platelets, leukocytes, granulocytes, monocytes and CD 34 + / mm3 and the following platelet growth factors: growth factor derived from platelets (PDGF), transforming B factor (TGF-B), Insulin like- 1 growth factor (IGF-1) and vascular endothelial growth factor (VEGF) using specific kits of enzyme-linked immuno-assay (ELISA). Measurements were made at baseline, prior to treatment, and serological PRPs obtained in patients 24h after administration. The route of injection was varied: intraarticular, intravenous, intramuscular or subcutaneous. The most consolidated techniques in literature for obtaining PRP were utilized to compared their performance with ours: [3,4]. Descriptive statistical calculations for the interpretation of analytical data were applied in each case.
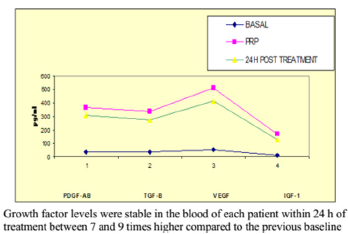
Figure 1: Average levels of growth factors in Alcaraz, Oliver and col technique measured at baseline, PRP and 24 h after the treatment.
No correlation between the amount of concentrated platelets and the amount of growth factors finally obtained was observed (Figure 1). The protocol set forth concentrated levels of platelets and leukocytes approximately 3 to 5 times higher than baseline levels with a predominance of mononuclears (75-90% of the white cellularity), up to 1-3% of them positive for marker CD 34+ without examining changes in cell counts practically safe increase between 150 and 300 times higher in the determination of CD 34 + at 24 hr post administration of PRP baseline count, as can be seen in Figures 2-4 respectively; and a levels of growth factors from 7-10 times greater than the patient’s baseline levels, with little variation in them. The growth factor levels were stable in the blood of each patient within 24 h of treatment between 7 and 9 times higher compared to the previous baseline, visible in Figure 1, Compared with other procedures discussed in the literature; This method achieves concentration between 1.5 and 3 times more platelets in the final product, as we can see in Figure 5, with a purification of growth factors overall type VEGF and TGF-B clearly superior, visible in Figure 6.
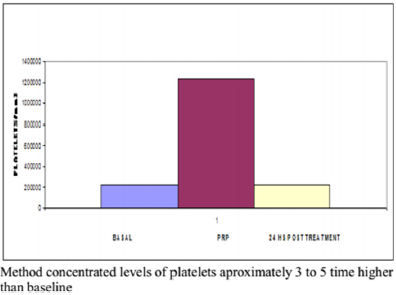
Figure 2: Half platelet counting in Alcaraz, Oliver et al. technique at baseline, PRP and 24 h after treatment.
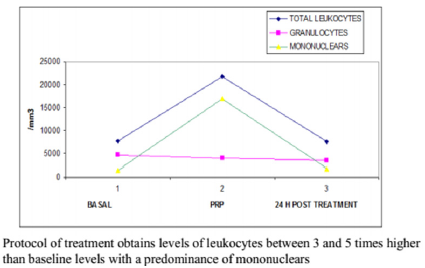
Figure 3: Half leukocitary (granulocitary and mononuclear) counting in Alcaraz, Oliver and col technique at basaline, PRP and 24 h after treatment.
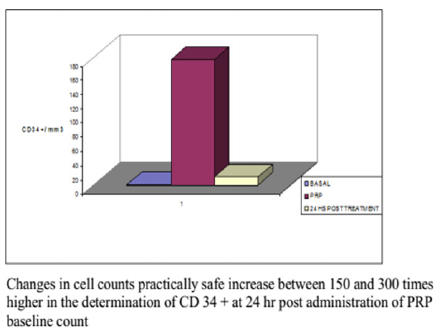
Figure 4: Half CD34+ counting in Alcaraz. Oliver and col technique at basaline, PRP and 24 h after treatment.
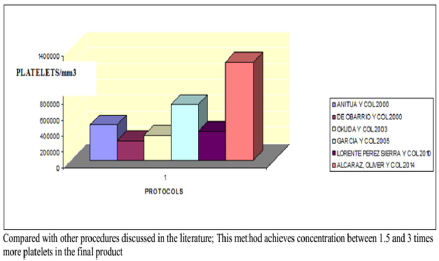
Figure 5: Average count of platelets in the PRPs of 6 procedures examined.
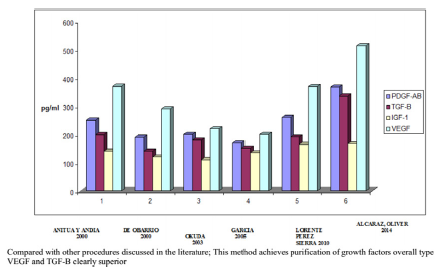
Figure 6: Average concentration of growth factors in the PRPs of 6 procedures examined.
According to the results, the proposed method focuses a greater amount of platelets as compared to other treatment protocols consulted if not correlated with the amount of growth factors obtained regardless of the age and sex of the patient [3,4], Consistent with the above previously by other authors. If it is noteworthy that in our PRPs that are rich in leukocytes there is greater amount of platelets and growth factors overcoat TGF-B and VEGF, compared to other procedures in which the leukocyte-depleted fraction has the final product obtained, as already proposed in previous studies, which can be explained by the presence of as many platelets in the border area leuco-platelet spinning [5,6]. Another fact to note is the presence of levels of platelet and plasma factors stable growth at 24 h post-treatment blood of very similar to the corresponding PRPs focused on patients, which could be explained by the high capacity of diffusion through of different tissues having these proteins, regardless of the means used to manage [3]. Similarly, review the mobilization capacity they have on CD 34+ [4,5], detecting blood higher figures at 24 h post-application compared to baseline count.


