Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
José M F Ferreira* and Avito Rebelo
Received: August 16, 2017; Published: September 07, 2017
Corresponding author: José M F Ferreira, Department of Materials and Ceramic Engineering, CICECO, University of Aveiro, 3810-193 Aveiro, Portugal
DOI: 10.26717/BJSTR.2017.01.000335
The research efforts are often driven by real needs for new or better materials, processes or systems that do not satisfactorily fulfil the expected/desired roles or functions. The discovery of bioactive glasses (BGs) in the late 1960s is a paradigmatic example of a new class of materials intended to replace inert metal and plastic implants that were not well tolerated by the body. Bioactive glasses elicit special responses when in contact with biological fluids that lead to the formation of a bone-like hydroxyapatite (HA) layer at the surface of BGs in vivo and to their strong bonding to living tissues. This last feature of 45S5Bioglass® represents a remarkable milestone and has inspired many other investigations aiming at further exploring the in vitro and in vivo performances of this or other related BG compositions. The main drawbacks of 45S5 Bioglass® include the high pH environment created by its high sodium content turning it cytotoxic; and the poor sintering ability that makes the fabrication of porous 3D scaffolds difficult. All these relevant features strongly depend on a number of interrelated factors that need to be well compromised, including the chemical composition and glass structure, which determine the biocompatibility, the degradation rate, and the easiness of processing (shaping and sintering) the material. The present work gives an overview about the motivations behind the development of a new series of alkali-free bioactive glass compositions that offer a set of well-balanced overall properties for the most demanding applications in healthcare, bone regeneration and tissue engineering.
Keywords: Alkali-Free Compositions; Biocompatibility; Processing Ability; Osteoinduction; Osseointegration
The discovery of bioactive glasses in the late 1960s is an excellent example of a target oriented research led by Larry L. Hench as an assistant professor at the University of Florida. The idea for the material was stimulated by a challenging discussing between him and the Colonel Klinker during a bus ride towards the U.S. Army Materials Research Conference held in Saga more, New York, in 1967 [1]. Klinker had recently returned to the United States after serving as an Army medical supply officer in Vietnam and was concerned with the number of amputations derived from the body’s rejection of inert metal and plastic implants. There was an urgent need for a novel material that could form a living bond with tissues in the body. Soon after, Larry L. Hench submitted a proposal to the U.S. Army Medical Research and Design Command that was funded in 1968 [1]. The bonding ability of a glass composition (45SiO2–24.5Na2O–25.5CaO–6P2O5 wt. %, called 45S5 Bioglass®) to bone and muscle after six weeks post implantation in rats was firstly observed in 1969 by Ted Greenlee, an Assistant Professor of Orthopaedic Surgery at the University of Florida and reported in papers in 1971 [2] and 1973 [3].
The 45S5 Bioglass® has been in clinical use since mid-1980s, with the first successful surgical use in the replacement of ossicles in middle ear [4,5]. It is also being marketed in particulate form under the trade name of Perioglas®, used to fill periodontal bone defects, and more recently as injectable pastes and putties under the trade name of Nova Bone®. According to a recent publication, 45S5 Bioglass® has been clinically applied in more than 1.5 million patients [6]. Even though, this composition presents several drawbacks derived from its high alkali content, including:
a. High dissolution rate [7] that causes fast resorption and may negatively affect the balance of natural bone remodelation, leading to gap formation between the tissue and the implant material [8];
b. Poor sintering ability and early crystallization [9-15] due to the close proximity between glass transition temperature (Tg~550ºC) and the onset of crystallization (Tc~610ºC) hindering densification and resulting in poor mechanical strength, a serious limitation for manufacturing highly porous scaffolds;
c. The cytotoxic effect caused by high doses of sodium leached to the culture medium [16,17].
The interest in bioactive glasses has been continuously increasing, attempting to further explore the properties of 45S5 Bioglass®, expanding the potential applications and overcoming some of its main drawbacks. The number of papers published per year in the field has noticeably increased especially from the beginning of 1990s, as can easily be observed in (Figure 1) that plots data compiled in a recent (July 2017) literature search in the Scopus.
Most of the reported bioactive glass compositions investigated so far [18-24] are inspired in the 45S5 Bioglass® and contain significant amounts of alkali oxides (Na2O, K2O) that decrease the melting temperature of the glass, but can reduce their performances in vitro and in vivo. The sudden release of alkali ions from alkali-containing BGs to the cell culture media was reported to exert in vitro cytotoxic effects [16,17]. On the other hand, the mismatch between the high rates of dissolution and degradation of 45S5 Bioglass® and the growth of new bone was confirmed in a study performed in rabbits [18- 24]. Such mismatches are likely to compromise bone regeneration especially in defects with critical size.
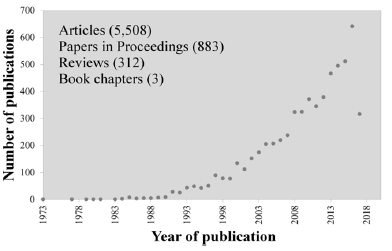
Figure 1: Number of papers published per year in the field of bioactive glass along the last 44 years.
In an attempt to overcome the above referred limitations of the alkali-containing glasses, a new series of alkali-free bioactive glasses compositions were designed based on a completely different concept, namely starting from the compositions of minerals that are biocompatible and bioactive, including diopside (DiCaMgSi2O6), fluorapatite (FACa5(PO4)3F), and tricalcium phosphate (TCP3CaO. P2O5) combined in different proportions. Figure 2 shows most of the investigated compositions in this ternary system, as well as in the binary Di-FA and Di-TCP ones [25-29].
The compositions corresponding to black symbols (λ) were less prone for glass formation and underwent fast crystallisation even upon quenching the melts in cold water to obtain the glass frits. The compositions corresponding to light grey symbols (λ) enabled obtaining amorphous frits, but the bulk glasses cast on metal plates tended to partially crystallise, especially in the parts farer from the metal plates that cooled more slowly. The most interesting compositions from the processing view point were those corresponding to dark grey symbols (λ).
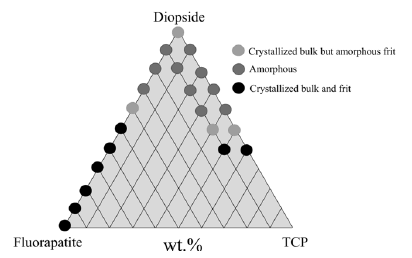
Figure 2: Graphical representation of the alkali-free bioactive glass compositions investigated [25-29].
In a sub-series of compositions, MgO was partially replaced (1-10 mol %) by ZnO [30], while in another sub-series CaO was partially replaced (1-10 mol %) by SrO [31]. In a third sub-series of compositions, these partial oxide substitutions were combined in an equimolar basis [32,33]. The effects of such individual or combined substitutions on the relevant properties of the resulting bioactive glasses were investigated and reported in several publications [30-33].These alkali-free bioactive glasses revealed to have several distinctive features including: moderate degradation rate, accompanied by a fast bio mineralisation rate in vitro with the formation of a hydroxycarbonate apatite (HCA) surface layer after 1 h of immersion in simulated body fluid (SBF) [26,30,32]; the ability to reduce oxidative stress [30,32]; osteogenic activity, inducing the differentiation of hMSCs into bone forming cells even in the absence of osteogenic medium [29]. This osteo induction effect was significantly greater in comparison to that of 45S5 Bioglass® [34]; and excellent bone bonding ability confirmed by in vivo studies using sheep as animal model [35].
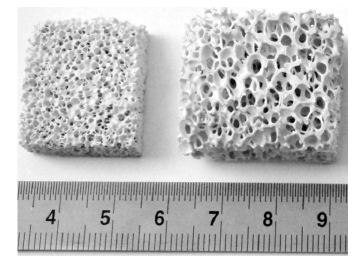
Figure 3: Porous scaffolds with different pore sizes fabricated by the polymeric sponge replication method from the composition 70-Di-10FA-20TCP.
Other interesting features of these bioactive glass compositions are the easiness of dispersing the glass powder frits in aqueous suspensions and scaffold fabrication by additive manufacturing techniques [36]. The pH values of the suspensions are lower in comparison to those of 45S5 Bioglass® and the particle size of powder frits can be more conveniently reduced and expose a larger surface area to the dispersion liquid without undergoing a so extensive dissolution [37,38]. Figure 3 shows porous scaffolds with different pore sizes fabricated by the polymeric sponge replication method from the composition consisting of 70 wt.% diopside (Di-CaMgSi2O6) - 10 wt.% fluorapatite (FA-Ca5(PO4)3F) - 20 wt.% tricalcium phosphate (TCP-3CaO.P2O5), or shortly, 70-Di- 10FA-20TCP. The scaffolds can be prepared with any desired size, contrarily to what happens in case of Bioglass 45S5®.
Still more important from the overall processing point of view is the excellent sintering ability, achieving full densification before the onset of crystallization, resulting in strong mechanical properties [25-28,31-33]. Figure 4 compares porous scaffolds fabricated by robo casting, an extrusion based additive manufacturing technique that enables designing not only the external form and dimensions, but also tailoring the internal structure. The starting materials were 45S5 Bioglass® (Figure 4a) [37,38], and our alkali-free bioactive glass 70-Di-10FA-20TCP (Figure 4b) [36].
It can be seen that the sintered rods of 45S5 Bioglass® are still too porous due to its poor sintering ability and early crystallization [9-15], leading to unsatisfactory compressive strengths values. In contrast, the compressive strength values of the scaffold fabricated from 70-Di-10FA-20TCP (Figure 4b) were significantly higher in comparison to those reported for cancellous bone (2-12 MPa) [36-38].
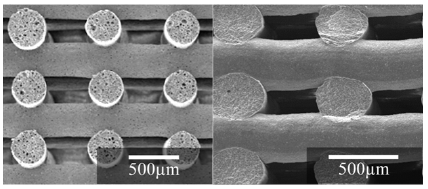
Figure 4: Comparison of the densification extent of the cylindrical rods deposited by rob casting from 45S5 Bioglass® (a), and from 70-Di-10FA-20TCP (b).
Larry Hench’s pioneering work can never be overemphasized. He was always a very creative and resourceful man and used to share his findings with generosity. We are fortunate enough being able to learn all his teachings about bioactivity and bioactive glasses. But his openness to new challenges is, by chance, the greatest of all his teachings. So, the best tribute we can give him is feeding ourselves with the full table of knowledge left by him and gaining strength to continue the journey in the search of better materials for healthcare applications. Our efforts put forward in the development of alkali-free bioactive glass compositions that could overcome the main drawbacks presented by 45S5 Bioglass® and other alkali-containing bioactive glass compositions went in that direction. From the results reported in our previous related publications one can conclude that they offer a set of wellbalanced overall properties for the most demanding applications in healthcare, bone regeneration and tissue engineering. The most salient features concerning the in vitro and in vivo performances include:
a. Absence of cytotoxic effects (no harmful dissolution products and the resulting pH);
b. Non-genotoxic – no damage to genes within a cell or DNA mutations;
c. Biocompatible – absence of any foreign body reaction;
d. Osteo conductive – bone readily grows on its surface;
e. Osteo inductive – recruits immature cells and stimulates them to develop into pre-osteoblasts, essential in any bone healing process;
f. Osseo integration – stable anchorage of an implant achieved by direct bone-to-implant contact.
Furthermore, our alkali-containing bioactive glass compositions are easy to process – an essential feature for scaffolds fabrication (derived from the moderate pH of the suspensions and the excellent sintering ability), achieving full densification before the onset of crystallization and conferring to the constructs adequate mechanical properties for the intended applications. All these features mean that significant progresses were made towards an ideal bioactive glass.
Thanks are due to CICECO-Aveiro Institute of Materials (UID/ CTM/50011/2013) project funded by FEDER funds through the Operational Programme Competitiveness Factors (COMPETE 2020) and the Portuguese Foundation for Science and Technology (FCT). The authors also would like to thank FCT (Portugal) by the financing of PEst-UID/NEU/04539/2013 and FEDER-COMPETE (FCOMP-01- 0124-FEDER-028417 and POCI-01-0145-FEDER-007440).


