Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Liu Yiwei, Sun Ke and Tang Jianguo*
Received: August 14, 2017; Published: August 30, 2017
Corresponding author: Tang Jianguo, Department of Otolaryngology-Head and Neck Surgery, Sir Run Run Shaw Hospital, Medical College of Zhejiang University, Hangzhou, 310016, China
DOI: 10.26717/BJSTR.2017.01.000311
Sturge-Weber syndrome (SWS), also known as encephalotrigeminal angiomatosis, was reported by Schirmer, Sturge and Weber in succession in mid-19th century. And it is a neurocutaneous disease classically presenting with a facial portwine stain in the ophthalmic distribution of the trigeminal nerve, glaucoma and vascular eye abnormalities, and an ipsilateral occipital leptomeningeal angioma. It is possible to observe nose and sinus involvement in SWS [1]. And there already have been some cases about SWS accompanying with chronic rhinosinusitis [2], but to our knowledge, there are still no cases describing SWS combining with ipsilateral chronic rhinosinusitis and unusual nasal polyps. Here, we report a case of SWS which incorporated with ipsilateral chronic rhinosinusitis and nasal polyps-more precisely hemangioma-liked nasal polyps.
Keywords: SWS: Sturge-Weber Syndrome; RBC: Red Blood Cells; HB: Hemoglobin; FESS: Functional Endoscopic Sinus Surgery; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; SPEDT: Single Photon Emission Computed Tomography; VAS: Visual Analogue Scale; FESS: Functional Endoscopic Sinus Surgery
A half year ago, a 40-year-old Chinese man known to have SWS visited our department complaining of progressive nasal obstruction, recurrent purulent nasal discharge and anosmia many years. He had been treated with no success using antibiotics, topical nasal decongestants and steroid nasal sprays. He had a history of left-sided epilepsy (but he had been seizure-free for many years without any antiepileptic therapy), headache, right buphthalmos and glaucoma which led his right eye to blindness, and progressive left hemiparesis. Fortunately, he was not severe mental disabled, and could look after himself and communicate with others basically. There was no family history of epilepsy, mental retardation or a neurocutaneous syndrome.
Physical examination showed a unilateral capillary malformation of the right face, upper neck (some parts even cross the midline), tongue, lateral pharyngeal wall, epiglottis and arytenoid area (Figure 1A-1C). Also right buphthalmos was found with exotropia 15-20° and blindness. Right nasal cavity which was full of polypoid tissue covered by mucopurulent discharge was observed and configured by nasendoscopy. In addition, muscular atrophy, muscle strength III-IV and hemiparesis were discovered in his left limb, as well as Babinski sign (+).
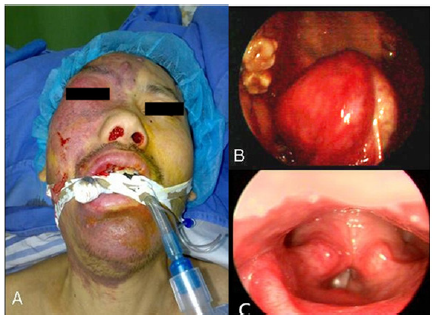
Figure 1: A-C Unilateral capillary malformation of the right-sided face, upper neck (some parts even cross the midline), tongue, lateral pharyngeal wall, epiglottis and arytenoid area.
CT scan displayed deformations of right sided skull, atrophy of right cerebrum, “tram-line” cortical calcification of right apical-occipital region, hypertrophy of right choroid plexus in the ventricle, and right buphthalmos. Also, low-density soft tissues (suggestive of nasal polyps) in the right nasal cavity, maxillary and enlarged frontal sinuses were observed in the CT scan photographs. Although the patient had no hearing problems, the enlarged right mastoid process was showed clearly (Figure 2A-2B). Ophthalmologic examinations presented that the right intra-ocular tension and visual acuity were 41.7mmHg and 0 respectively, while the left were 12.0mmHg and 1.0. The degree of mental retardation was evaluated through Wechsler Intelligence.
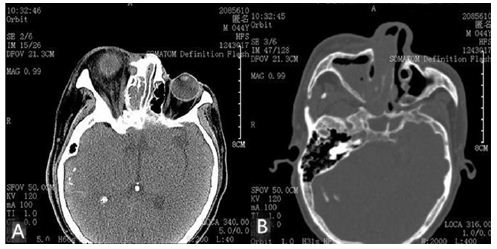
Figure 2A: Right buphthalmos, and hypertrophy of the
right choroid plexus in the right ventricle.
Figure 2B: Right maxillary and nasal cavity filled with
low-density tissue, enlarged right mastoid process and
thickened diploe of the right skull.
After excluding surgical contraindications, a unilateral functional endoscopic sinus surgery (FESS) under general anesthesia was performed. A lot of excessive proliferated polypoid tissue in the nasal cavity and sinuses were observed during the operation. What attracted us most was that the quantity of intraoperatie bleeding was much more than those with ipsilateral or unilateral nasal polyps, even those with more severe bilateral nasal polyps in our experience, although conditions of the operation such as blood pressure controlling, operative time were almost the same. The routine blood test showed us that the red blood cells (RBC) decreased from 4.68×1012/L (the last preoperative day) to 2.95×1012/L (the first postoperative day), and hemoglobin (Hb) fell from 140g/L (the last preoperative day) to 89g/L (the first postoperative day). All these above gave us some information that there may be any connections between intraoperative bleeding and SWS. Therefore, the pathological features of the nasal polyps became the key point. In the end, hemangioma-liked nasal polyps were observed in microscopical pathological examination (Figure 3). 6 months follow-up showed good ventilation and reduced purulent nasal discharge without late complications or recurrence.
SWS is really a rare disease with the prevalence actually estimated at 1/50,000. The almost entirely sporadic and focal nature of SWS (equal incidence in men and women; rare familial cases, especially lack of clinical similarity in monozygotic twins) indicates that the presence of a somatic mutation [3], however, somatic activating mutation in GNAQ was proved to be the etiology of SWS in 2013 [4]. Besides the three classic clinical features-facial port-wine stains, glaucoma and ipsilateral leptomeningeal angioma, epilepsy in early life, vascular headache, hemianopsia, developmental delay and mental retardation, contralateral hemiparesis and hemisensory disturbance, gingival and mucosal hypertrophy, skeletal overgrowth, and tram-line intracranial calcifications can also be found [5]. The diagnosis of SWS is mainly on the clinical features and imaging data such as computed tomography (CT) and magnetic resonance imaging (MRI) which are the most widely used. And also positron emission tomography (PET) and single photon emission computed tomography (SPECT) that can demonstrate hypoperfusion and cortical hypometabolism in corresponding area are complementary investigations [6]. However, the key diagnostic standard in SWS is the intracranial leptomeningeal angiomatosis in histology. Transcranial Doppler Ultrasound is mainly used to predict neurological progression and assess the therapeutic effect [7] and the size of facial port-wine stain also can be used to evaluate the eventual neurologic disease severity in SWS patients with brain involvement [8]. Today, these are still no effective ways to heal SWS, lifelong medical treatments coupled with frequent surgeries are recommended [5]. The prognosis of SWS varies widely, presence of seizure and bilateranial leptomeningeal angiomatosis are associated with poorer clinical outcomes [9]. In our case, the patient who consisted of facial port-wine stain, leptomeningeal angioma and glaucoma obviously belonged to type I according to the classification of Roach Scale [10].
A nasal polyp is now considered as a subgroup of chronic rhinosinusitis, called chronic rhinosinusitis with nasal polyps. The treatment option for chronic rhinosinusitis with nasal polyps is either medical or surgical. According to visual analogue scale (VAS) score, additions of different courses of topical nasal steroids even oral steroids are provided to help control symptoms firstly. If conservative treatment fails, functional endoscopic sinus surgery (FESS), based on logical physiological concepts, aiming to restore ventilation and mucociliary clearance of the nasal sinuses is performed. Follow-up nasal douching, topical steroids, and longterm antibiotics after operations are also emphasized [11].
As far as we know, there are no references that have demonstrated evidently the connection between SWS and chronic rhinosinusitis, let alone chronic rhinosinusitis with nasal polyps. However, deformed structures, as well as overgrowth of soft tissue of noses and sinuses caused by SWS may be considered as predisposing factors. We report the first patient who had type I SWS with ipsilateral chronic rhinosinusitis and hemangioma-liked nasal polyps. Therapeutic regimen of such a case, similar to that of usual unilateral chronic rhinosinusitis with nasal polyps, is functional endoscopic sinus surgery (FESS). In addition, on the basis of hemangioma-liked nasal polyps and much more intraoperative blood, Yamashiro’s experience of successful managing bleeding of a patient with SWS undergoing oral surgery by relieving the stress preoperatively, suppressing elevation of blood pressure and using hemostatic agent intraoperatively could be worth profiting from [12].
A rare case of SWS with ipsilateral chronic rhinosinusitis and hemangioma-liked nasal polyps is presented. The relationship between SWS and nose and sinus is poorly understood, though involvement of nose and sinus is common. Therapeutic strategy is the same as that of chronic rhinosinusitis with nasal polyps according to European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS). However, intraoperative and preoperative blood should be paid an attention to. Maybe perfect preoperative preparation, intraoperative and preoperative hemostasis should be emphasized, including histological biopsy.


