Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Aneela Zameer Durrani1*, Muhammad Usman Raza1 and Aijaz Ali Channa2
Received: August 08, 2017; Published: August 28, 2017
Corresponding author: Aneela Zameer Durrani, Department of Clinical Medicine and Surgery, University of Veterinary and Animal Sciences, Pakistan
DOI: 10.26717/BJSTR.2017.01.000303
Antimicrobial resistance (AMR) is major problem in treatment of uterine infections in dairy animals. To suggest an alternative treatment, avoiding antibiotics, the effect of ozone as therapy against common bacterial uterine infections in dairy females the present study was carried out in 50 animals at dairy farm in district Sarghoda, Punjab province, Pakistan. The animals with history of repeat breeding and abnormal uterine secretions were selected and randomly divided in two groups. Group A received commercially prepared Ozone(Sanofoam®) intrauterine treatment while group B wasgiven gentamicin sulphate(Gentafar 10%®)@30gm in 1000 ml saline water treat intrauterine. Uterine lavage was taken twice, once before applying treatment and second after 8 hours of applying treatment. Samples were cultured for bacteriology to detect E.coli, F. necrophorum, A. pyogenes and St. pyogenes. Bacteria wise percentage of uterine infection was highest for E.coli (88%) followed by 84%, 68% and 60% for A. pyogenes, F. necrophorum, and St. pyogenes respectively. Difference of the colony forming units before and after applying both treatments for each bacterium was calculated. Results were interpreted statistically. Differences among the groups were considered significant at P < 0.05. E. coli, F.necrophorum and St. pyogenes were highly significant as the P value for group differences was less than 0.05. Group differences among St.pyogenes showed no significance as the P>0.05. The results showed that intrauterine treatment with ozone was more responsive (38/50, 76%) as compared to gentamicin sulphate intrauterine treatment (37/50,74%) in cross bred dairy cows with bacterial infections.
Keywords: Ozone; Uterine infections; Dairy female; Bacteriology; Efficacy
Efficient fertility of lactating dairy cows has always been the doorstep to economically profitable dairy farming. The reproductive efficiency of a cow after parturition in a herd is associated with many factors such as parity, body weight (BW) loss, the number of days in and without milk production, heat stress, season during which artificial insemination (AI) is done, seasonal condition during conception, bull being used for the AI, and the technician who performs the artificial insemination [1]. Most of the dairy farmers are not able to achieve targets for reproductive performance and bear economic closes.
Postpartum uterine infections are generally main reason to insemination failure and have traditionally occupied a great amount of veterinarians’ attention [2] it is mostly agreed that uterine diseases in the cow after parturition have a negative effect on overall reproductive performance. In the past few decades, the knowledge of the Pathophysiology for diagnosing clinical abnormalities e.g., metritis, endometritis, and subclinical endometritis (SE) has been increased significantly [3] Most of the times, these postpartum reproductive disorders have same etiology and interlink with each other [4].
Antimicrobial resistance is a growing veterinary health concern globally. When dairy animal is infected with resistant bacteria, not only treatment of that animal becomes more difficult, but the antibiotic-resistant bacterium may spread to other animals at farm. The treatment failure with antibiotics results in prolonged illnesses with, more complications, more vetenarians visits and use of stronger and more expensive drugs along with more deaths caused by bacterial infections (FDA, 2016). A wide range of bacteria has been recovered from uterus of postpartum cows. Besides Actinomvcespvogenes, certain Gram-negative anaerobic bacteria like Fusobacteriumnecrophorum and Bacteroides Spp. May have a key role in repeat breeding in dairy cows [5] these bacteria are responsible for repeat breeding in cows. Repeat breeder cows are characterized as cows having regular estrus cyclesi.e of 17 to 25 days and have history of three or more artificial inseminations (AIs) without conception [3].
Ozone helps in stimulation of lymphocytes and monocytes toassist in release of several cytokines which enhance the tissue regeneration mechanism and initiate the processes of tissue granulation and epithelial formation. Ozone breaks through the microorganism (bacteria and germs) cell membrane, and also destroys viruses by diffusing through the protein coat in the nucleic acid core, resulting in damage of the viral nucleic acid. Commercial ozone preparations include foam, pearls, boluses, injections, cream and palettes, etc. [6]. Indiscriminate use of antibiotics use in reproductive disorders in dairy animals have developed some antimicrobial resistant That in turn threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria. Keeping in view the antimicrobial resistance (AMR) in uterine infections the present study was designed to compare the efficacy of ozone and gentamicin sulphate on uterine infections in crossbred dairy cows so that alternative effective therapy may be suggested to overcome antibiotic resistant bacteria in dairy animals.
Fifty cross bred (Local × HF) dairy cows were selected randomly at dairy farms having history of repeat breeding as well as abnormal uterine secretions. These animals were in good body condition, being fed proper nutrition and free from any apparent disease. Animals were divided into two groups (25 animals each).
Group A: This group was treated with commercially prepared Ozone intra uterine.
Group B: This group was treated with Gentamicin sulphate intrauterine (1000 mg in 30 ml normal saline).
For representation of results, differences of the before and after treatments were taken and groups were named as following
A. dAE: difference of E.coli CFUs before and after treatment in A group.
B. dAF: difference of F. necrophorum before and after treatment in A group.
C. dAA: difference of A. pyogenes before and after treatment in A group.
D. dAS: difference of St. pyogenes before and after treatment in A group.
E. dBE: difference of E. coli before and after treatment in B group.
F. dBF: difference of F. necrophorum before and after treatment in B group.
G. dBA: difference of A. pyogenes before and after treatment in B group.
H. dBS: difference of St. pyogenes before and after treatment in B group.
Samples of uterine fluids were collected twice from each animal. First sample was taken before applying the treatment and second sample was taken after eight hours of treatment as the half life of gentamicin sulphate following intrauterine application is 2 hours [7]. After restraining, animal’s vagina was washed by using 1% KMnO4 solution to lower the vaginal floor contamination. Single packed A.I sheath was introduced in the uterus through cervix of the cow with the help of A.I gun. Once the sheath was introduced in the uterus, A.I gun was removed while keeping the sheath inside the uterus. A syringe containing 30 ml of normal saline was attached with the sheath and this normal saline was introduced in the uterus of the cow. Uterus was massaged for 10 to 15 seconds. A 10 ml disposable syringe was attached with the sheath and uterine lavage was taken in it by aspirating the fluid from uterus. Syringe containing the sample was recapped immediately and was tagged according to cow’s tag number. Freshly collected samples were stored in the refrigerator to prevent sample spoilage.
Bacterial culture: Samples were processed on agar medium for bacteriology. For each bacterium separate medium was used. E.coli was grown on MacConkey agar under aerobic condition in the incubator at 37ºC temperature. On MacConkey agar E. coli specimen yielded characteristic pink colonies. F. necrophorum was grown on laboratory nutrient agar which was supplemented with 5 % sheep blood under anaerobic conditions in incubator at 37ºC. Small rounded, convex and translucent colonies appeared on blood agar showing few hemolysis as well. A.pyogenes was grown on laboratory nutrient agar which was supplemented with 5 % sheep blood under anaerobic conditions in incubator at 37ºC. St.pyogenes was grown on nutrient agar which was supplemented with 5 % sheep blood under anaerobic conditions in incubator at 37ºC. St.pyogenes caused beta hemolysis which resulted in complete lysis of red blood cells and hemoglobin. This resulted in complete clearing of the blood around the colonies.
Bacterial staining: Gram’s staining was done to distinguish gram positive and negative bacteria. Gram positive bacteria on Gram’s staining appeared purple while gram negative bacteria were appeared pink under microscope by using oil emersion lens [8] Confirmatory biochemical tests were done to confirm bacteria.
Colony forming units: Colony forming units were counted for each bacterium by using magnifying glass. These were calculated to compare the findings of both drugs used. To count colony forming units 1ml of original sample was diluted in 99ml of buffer solution. This dilution was used to grow bacteria grown on respective growth medium by pour plate method in incubator under standard conditions i.e temperature and time. Colony forming units were calculated by following formula; CFUs/ml = (colonies observed / 1) × 102
Percentage of positive cases: Percentage of positive cases for each bacterium was calculated by following formula; Percentage of positive cases = (No. of positive cases)/(Total no. of sample)×100
Therapeutic trials: Group A received intrauterine Ozone (Sanofoam®) treatment by same sheath which was used for collecting the sample without retrieving it out. Sanofoam® bottle pressure button was pressed for 5 seconds as per company’s recommendation. Group B received 1000mg of Gentamicin sulphate (Gentafar 10 %®) treatment by same technique applied for A group treatment. Uterine samples were collected again following eight hours post treatment from each animal by using same protocols as described above. Ethical approval has been granted by ORIC office of UVAS in monthly meeting for this study
Statistical analysis was done with Statistical Packages for Social Sciences (SPSS Inc.) for the windows Version 13.3 (Chicago IL, USA). The Levene’s test was applied to test the equality of the variances. The data were analyzed using independent two samples T-test. The Group differences were compared by the Duncan’s Multiple Range Test [9] Differences among the groups were considered significant at P < 0.05.
Gram Staining: On Gram’s staining E.coli (Gram negative)were characteristic rod shape, F. necrophorum (Gram negative)were short cocco-bacillus while A.pyogenes (Gram positive) appeared club-shaped bacillus and Stpyogenes (Gram positive) were round.
Confirmatory biochemical test for identified bacteria: E coli were Catalase (positive), Methyl reduction (positive), Indole (positive), Lactose fermentation (positive), Ribose fermentation (negative). F. necrophorum were negative for Glucose, Mannitol and Lactose sugar fermentation, Indole (positive), propionate (positive) and positive for lipase production on agar medium supplemented with egg yolk. A. pyogenes were starch fermentation (positive), lactose fermentation (positive), ribose fermentation (positive), urease (negative) and mannitol fermentation (negative). Sr. pyogenes were catalase (negative), PYR (positive), CAMP (negative), non motile, bacitracin sensitivity (positive) and bile solubility (negative).
All samples were processed for bacteriological examination and E.Coli, F. Necrophorum, A. Pyogenes & St. pyogenes were isolated. Results are summarized in (Table 1). Before treatment Group A had more percentage of E.coli positive cases i.e. 48% as compared to group B i.e 40%. Group B had higher positive percentage for A. pyogenes (44%) and St. pyogenes (32%) as compared to group A. Bacteria wise positive percentage among two experimental groups under field conditions is summarized in (Table 2). Colony forming units for each bacterium was calculated in the positive sample before and after treatment. The data is summarized in (Table 3 & 4).
Table 1: Bacteria wise Infection percentage of 50 animals before treatment.

Table 2: Bacteria wise positive percentage among two experimental groups under field conditions before treatment.
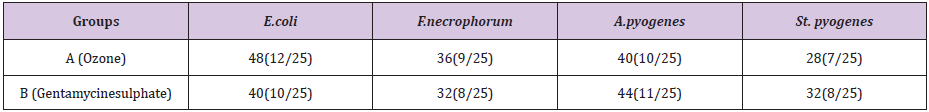
Table 3: Colony forming units/ml for Ozone -Group before and after treatment.
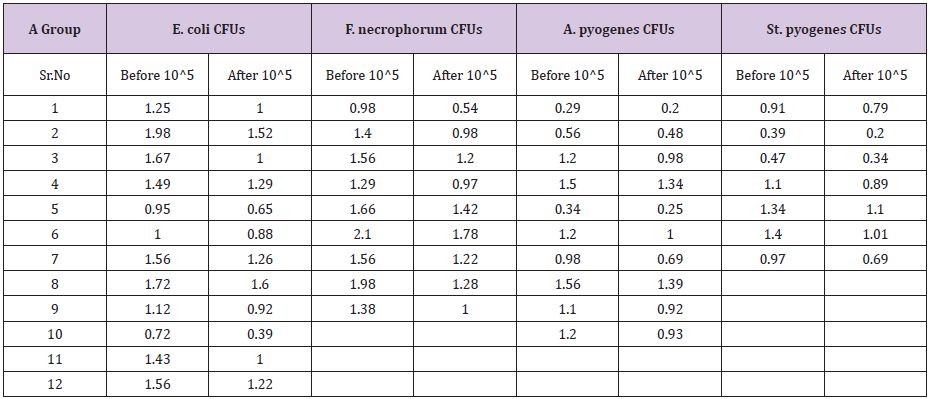
Table 4: Colony forming units/ml for Gentamycin-Group before and after treatment.
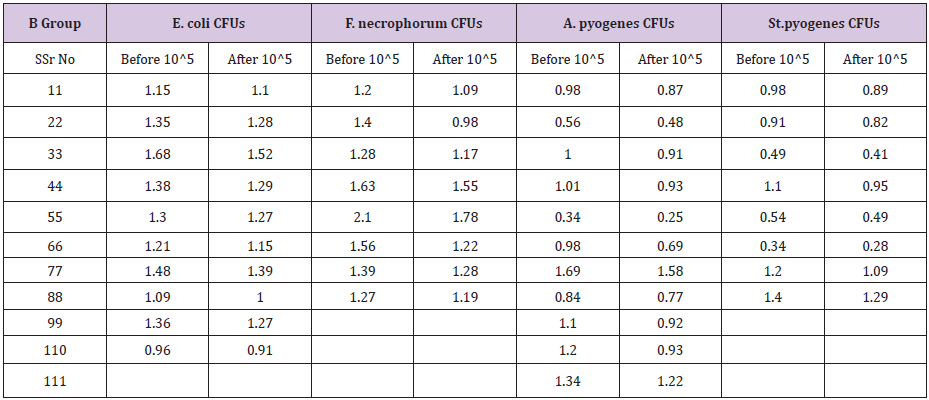
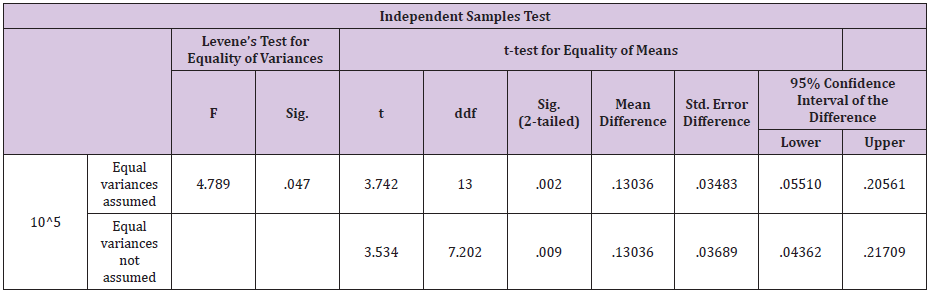
Bacteria wise data was statistically by Levene’s test for equality of variances by using Statistical Packages for Social Sciences (SPSS Inc.) for the windows Version 13.3 (Chicago IL, USA). Results of E. coli showed46% samples were positive for in group A as compared to 40% in group B before treatment .Statistical analysis of data for E.coli showed that the variances was not equal as the F-value was greater than 0.05. The results showed that mean of the difference in A group was greater than the mean difference of B group. The results were highly significant for A group i.e P <0.05 which shows that ozone is better in efficacy as compared to gentamicin sulphate in case of E.coli infection of the uterus.(Table 5) For F.necrophorum 36% samples were positive for in group A as compared to 32% in group B before treatment .
Table 5: Independent samples T - test results for E. coli.
Statistical analysis of data for F.necrophorum showed that variances was not equal as the F-value was greater than 0.05. The results were significant for ozone i.e P<0.05 which shows that ozone is better in efficacy as compared to gentamicin sulphate in case of F.necrophorum infection of the uterus (Table 6) 40% samples were positive for A.pyogenes in group A as compared to 44% in group B. Statistical analysis showed that mean of the difference in A group was greater than the mean difference of B group for this bacteria. The results were not significant for ozone i.e P > 0.05 which shows that gentamicin sulphate is better in efficacy as compared to ozone in case of A. Pyogenes infection of the uterus (Table 7). 28% samples were positive for St. pyogenes in group A as compared to 44% in group B before treatment. Statistical analysis showed that results were highly significant for ozone i.e P<0.05 which shows that ozone is better in efficacy as compared to gentamicin sulphate in case of St. Pyogenes infection of the uterus (Table 8).
Table 6: Independent samples T - test results for F. necrophorum.
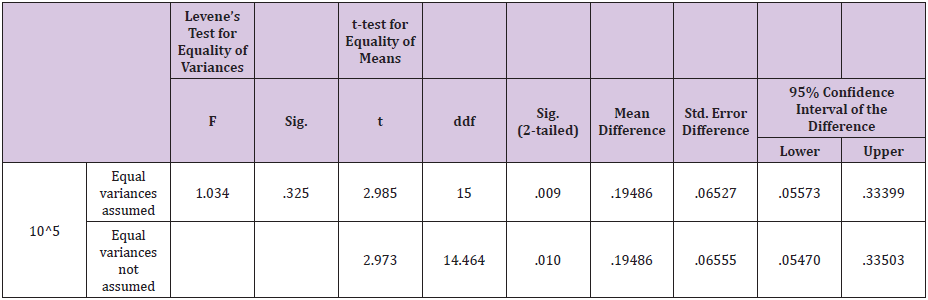
Table 7: Independent samples T - test results for A. pyogenes.
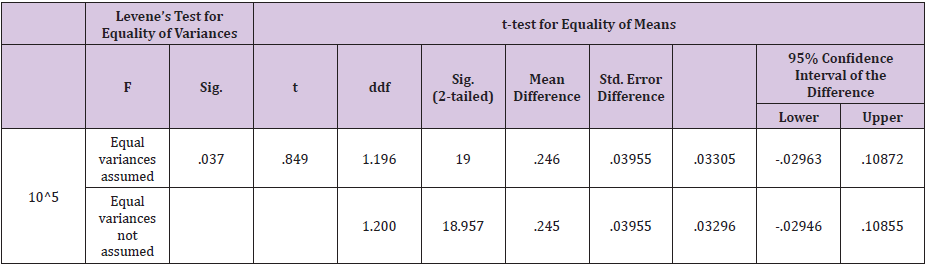
Table 8: Independent samples T - test results for St. pyogenes.
Uterus of the cow is much vulnerable to infections after parturition. Uterine infections as well as repeat breeding are the major constraints to dairy industry. In our study comparative efficacy was studied for ozone as an alternative therapy for antibiotici.e gentamicin sulphate under field conditions. The trial was conducted on 50 cross bred dairy cows on commercial dairy farms. The bacteria most commonly isolated from dairy cows, with uterine infections, were E. coli, F. necrophorum, A. pyogenes & St.pyogenes. E.Coli and A.pyogens were reported previously in uterine infections. Fusobacterium necrophorum and Bacteroides spp. are also recognized as opportunistic bacteria that can cause uterine infections as reported by [9] the most prevalent pathogens isolated in our study were E. coli and A. pyogenes. Percentage of E.coli and A.pyogenes in our study was 44 and 42 % respectively.
Similar findings were documented by [10] in his study and reported E.coli (37%) and A.pyogenes (49 %) in uterine infections [11]. however, reported 33.5 % of E.coli in uterine infections. E coli and A.pyogenesare also important from public health aspect as they can lead to illness and even fatality in humans. In this study percentage of St.pyogenes infection was 30% that was almost same (32%) as described by [12]. Streptococci bacteria cause can lead to serious infection, such as blood infection, , rheumatic fever if food items such as milk carries this pathogen. The results were significantly different for F.necrophorum (34%), in present study while Dohmen [12] reported 60 % cases of F necrophorum. F necrophorum can be a primary pathogen that can cause localized abscesses and throat infections in humans. Bocci [13].
In our study Ozone was more effective for E. coli than Gentamycin sulphate while A. pyogenes were more susceptible to Gentamicn sulphate than ozone treatment. Isolates of F.necrophorum and St.pyogenes were moderately susceptible to gentamycin sulphate and highly susceptible to the ozone treatment ( There were few investigations of intrauterine treatment as sequale to the retained fetal membrane in dairy goats and Holstein dairy cows by ozone [14] Findings in this study are the first reported data regarding the specifically Ozone treatment response of common bacterial pathogens under field conditions in uterine infections of cross bred dairy cows. Ozone assumed, assumed as a very potent oxidant, is one among alternative techniques to antibiotic therapy. The advantage of using ozone rather than antibiotics is to avoid bacterial resistance in consumers of foodstuffs of animal origin. Other advantage is that ozone has no withdrawal period for milk, meat and other products [13] Moreover, ozone acts both, as germicidal as well as fungicidal due to oxidative process of peroxides present on the microbial capsules that are destroyed by the ozone [15,16].
E. coli, F. necrophorum, A. pyogenes & St. pyogenes were isolated as predominant bacteria in dairy animals with intrauterine infection. Highest percentage of infection was recorded for E.coli followed by A. pyogens, F.necrophorum and St.pyogenes. The ozone treatment was more effective for E. coli, F. necrophorum and St. pyogenes as compared to Gentamycin sulphate. A.pyogenes, however, was more susceptible to gentamycine sulphate as compared to ozone treatment.
There is not any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could in appropriately influence, or be perceived to influence our work.


