Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
*Gabriel Levin and Henry chill
Received: August 07, 2017; Published: August 17, 2017
Corresponding author: Gabriel Levin, Department of Obstetrics and Gynecology, Hadassah University Hospital, PO Box 12000, Jerusalem 91120, Israel
DOI: 10.26717/BJSTR.2017.01.000276
Severe methotrexate neurological adverse event due to medical treatment of an ectopic pregnancy is presented. Severe adverse effects are rare among patiens treated for ectopic pregnancy with MTX. , it should be used with caution and severe toxicity should be kept in mind with high index of awareness for any symptoms development after the treatment.
Keywords: Methotrexate; Pregnancy; Ectopic; Toxicity
Abbreviations: MTX: Methotrexate; BHCG: Beta Humoen Chorionic Gonadotropin; MRI: Magnetic Resonance Imaging; CSF: Cerebro Spinal Fluid
Ectopic pregnancy is a serious, yet not uncommon condition in gynecologic practice. When treatment of an ectopic pregnancy is feasible by a medical means –the mainstay of treatment is by intramuscular injection of methotrexate (MTX) [1]. MTX treatment can be given by a single dose or by a multiple dose regimens. The single dose regimen is preferred usually due to is high efficacy, low adverse effect profile and low cost [2]. An adverse effect are usually mild and self-limited and has the incidence of about 10-30% [3]. Most common adverse effects are those effecting the mucosal tissue such as stomatitis and diarrhea. We hereby present a young healthy woman who had severe and rare neurological adverse event after single dose MTX treatment.
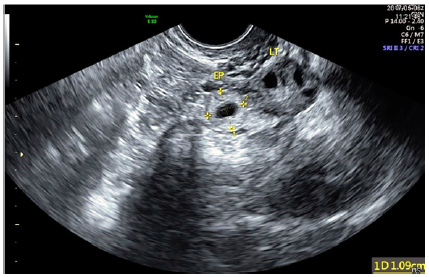
A gravida 2 26 years old healthy women was admitted to our department with symptoms of lower abdominal pain and a positive pregnancy test. Her medical history revealed a spontaneous abortion at 1st trimester which was treated medically by a course of vaginal misoprostol. Her bHCG level was 1826. A pelvic ultrasound examination revealed a 6 mm endometrial thickness and a hypo echoic mass measuring 10 mm near the left ovary surrounded by fluid (Figure 1). A second bHCG level was 2453 and a diagnosis of an ectopic pregnancy was made. The women was treated with an intramuscular injection of 78 milligram of MTX and discharged free of symptoms or pain. BHCG levels declined to a level of 294 on day 7. On the 12 day after the treatment she presented to the emergency department with symptoms of bilateral paresthesia of the lower limbs which ascended to the pelvis. The symptoms worsened and progressed cephalic.
Figure 1: Ectopic pregnancy by vaginal ultrasound.
A neurologic examination revealed a saddle anesthesia and lower limbs paresthesia. Her bHCG level was 73 and the gynecological examination was normal. A CT scan of the lumbosacral spine was done and was without abnormal findings. The woman was hospitalized for further evaluation due to her neurologic symptoms. During her hospitalization a neurological examination revealed decreased reflexes in lower limbs, decrease in the vibration sensation and decreased pain sensation up to the level of the umbilicus. A lumbar puncture was done and the CSF was found to be normal. Vitamin B12, Folic acid and homocysteine levels were examined and were found to be normal in the serum. All other laboratory examinations were normal including autoimmune serology and hyper coagulation.
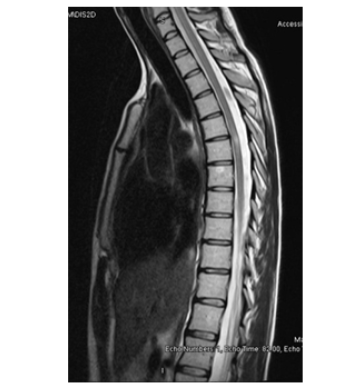
Due to deterioration in terms of neurological symptoms and signs a treatment with short intravenous course of high-dose methylprednisolone (1 gram a day for 5 days) was started. A whole spine MRI was done and revealed hyper intense T2 signal measuring 8 mm at level of T6 vertebrae in the spinal cord (Figures 2 & 3). A diagnosis of transverse myelitis was done. Treatment was continued with a methylprednisolone for 10 days with improvements of symptoms. The woman was discharged with tapering down course of oral prednisone.
Figure 2: Lesion at T6 level - transverse.
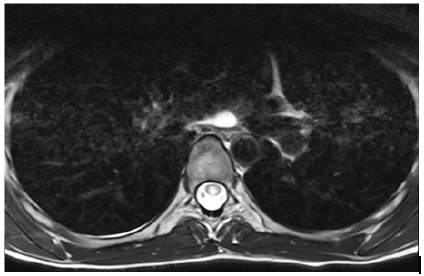
Figure 3: Lesion at T6 level – saggital.
MTX is the mainstay medical treatment for ectopic pregnancy. MTX is cheap and minimally toxic in low doses as given in these situations. Adverse effects can be seen more frequently in multi-dose protocols. The adverse effects of MTX are caused by irreversible inhibition of the enzyme dihydrofolate reductase in purine synthesis and the implication of this process on body tissues. The potential severe adverse effects of MTX treatment are hepatotoxicity, pulmonary toxicity, and risk of infection, myelosupression and nephrotoxicity. Severe toxicity is an unexpected condition [4-6]. Neurological adverse effects are rare and can be seen when MTX is given intrathecally to eradicate leukemic cells from central nervous system (CNS) in leukemia treatment protocols [4,5]. These neurological adverse effects include acute toxic leukoencephalopathy, seizures, headache, aphasia, hemi paresis or altered mental status [7].
In the present case we present previously unknown severe adverse effect myelitis of the spinal cord. A presumptive explanation is intrathecal absorption of the MTX from the muscular injection site to the CSF by route of intravascular penetration of the drug [8] and crossing of the blood-brain barrier. Fortunately, the patient’s signs and symptoms regressed while treatment with corticosteroids was started and maintained. In review of the literature, severe toxicity due to MTX treatment for ectopic pregnancy in a healthy woman has not been reported. In conclusion, unexpected toxicity with MTX should be kept in mind during use of this simple treatment method.


