Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Goran Pavlisa*
Received: July 08, 2017; Published: July 18, 2017
Corresponding author: Goran Pavlisa, Clinical Institute of Radiology, University Hospital Center, Zagreb - Rebro, Kispaticeva 12, 10000 Zagreb, Croatia, Italy
DOI: 10.26717/BJSTR.2017.01.000196
We present a series of 31 patients who underwent endovascular treatment due to a pericallosal artery aneurysm. To our knowledge, this is the largest series of patients with pericallosal aneurysms treated by endovascular approach reported so far. The distal location and generally small size of these aneurysms both make them difficult for surgical and endovascular treatment. Our aim was to try to define the risk factors for recanalization and complications of treatment. Various factors were analyzed, such as the patient’s age, occurrence of subarachnoid hemorrhage, aneurysm size, morphology/neck width and side of the aneurysm, incorporation of arterial branches, dominant ipsilateral A1 segment and other anatomic variations, presence of other aneurysms, type of coils used for treatment, the need for stent implantation and the initial degree of occlusion. Our rate of complications of 9.7% and recanalization of 16% were relatively high compared to other aneurysm sites. Relatively large aneurysms and those which incorporated branches were more prone to recanalization. Pericallosal aneurysms in our study were however very morphologically and anatomically heterogeneous, making our sample size insufficient to show the impact of these factors on complications.
Keywords: Pericallosal artery aneurysm; Endovascular treatment; Aneurysm recanalization; Complications
Distally located intracranial aneurysms are generally rare; the most commonly encountered are aneurysms at the junction of pericallosal and callosomarginal artery. They are usually referred to as pericallosal artery aneurysms (PAAs), or distal anterior cerebral artery (ACA) aneurysms. Neurosurgical approach in this location is difficult because of the proximity to falx [1] and typically small diameter of aneurysms at this site [2], with higher complication rate than in other aneurysm sites [3-6], although there are reports of unfavourable course even in proximal ACA aneurysms [7]. Distal ACA aneurysms may require complex treatment techniques [8].
Endovascular treatment is also complicated by distal location which impedes the manipulation of the micro catheter. Tortuosity of arteries proximal to the aneurysm, especially the angle at the origin of A1 segment of ACA and carotid siphon, further impedes the passage of the micro catheter and micro guide wire, sometimes with a necessary use of force during micro catheterization. This may result in an abrupt entrance into the small aneurysm, which may result in rupture. PAAs typically have a funnel-shaped neck which is difficult for embolization without compromising the flow in the callosomarginal artery origin. Neck remnant after coiling poses the risk for recanalization/re growth of the aneurysm.
Small lumen of ACA may even be obstructed by the micro catheter, so that we may be unable to visualize the aneurysm and anatomic landmarks at the neck at the moment of coiling. All these factors have been reported, with a relatively high rate of complications in endovascular management and high rate of recanalization [9-13]. To our knowledge, we present the largest series of 31 patients so far reported in the literature. We aimed to add to the present knowledge of endovascular treatment of PAAs and to define the risk factors for complications and aneurysm recanalization in this location.
This is a single-center retrospective study of 31 patients who have undergone endovascular repair of a pericallosal artery aneurysm, between 2005 and 2015. Endovascular procedures were performed on a single-plane digital angiography system (Axiom Artis FA; Siemens AG, Erlangen, Germany) with patients in general anesthesia. The patients with unruptured aneurysms received iv. heparin in a dose of 50 IU/kg; no heparin was administered in those with ruptured aneurysms. The follow-up was routinely performed 3 months after the treatment by 3D time-of-flight magnetic resonance angiography, and 6-12 months after the treatment by digital subtraction angiography (DSA).
The relationship between the rate of complications and aneurysm recanalization was investigated against the following factors:
A. Anatomical factors: aneurysm size, neck width, incorporation of arterial branches in the aneurysm neck/sac, anatomical variations, the side of the aneurysm and other aneurysms.
B. Technical factors: coil manufacturer, type of microcatheter, degree of initial occlusion (complete or incomplete) and implanted stent.
C. Clinical factors: subarachnoid hemorrhage (SAH).
D. Demographic factors: age, sex, year of treatment
The year of treatment was considered regarding operator experience, since endovascular practice in our institution started in 2003, and we hypothesized that patients treated in the earlier years of our practice may have had a higher rate of adverse events.
Statistical analysis was performed by Mann-Whitney test, correlation-contingency test and simple regression, using StatView 5.0 software. This paper has been written in accordance with ethical standards laid down by the declaration of Helsinki and after the appropriate insitutional clearance. All the patients gave their informed consent for enrollment in this retrospective research.
There were 26 female (84%) and 5 male patients, with an average age of 52, with a total of 37 procedures, including repeated embolizations in patients with significantly recanalized aneurysms. The incidence of PAAs in our study was 3%. Multiple aneurysms were found in 44% of the patients.
Complications and recanalization
Peri-procedural complications occurred in 7 patients (22.6%), however they were symptomatic in 3 patients (9.7%), presenting as ischemic stroke in the anterior cerebral artery territory due to thrombo-embolic events during the intervention (1 patient) and 5 days after the procedure (1 patient), and due to parent artery occlusion by coils (1 patient). Other 4 patients did not experience clinical symptoms; three of them had intra-procedural thromboembolic events and in one patient a procedural rupture of the aneurysm occurred (3%).
Aneurysm recanalization was encountered in 9 patients (29%) and in 5 (16%) of them it was considered significant to warrant a second interventional procedure. Recanalization occurred between 2 and 36 months after intervention. None of the patients experienced rupture of the aneurysm after endovascular therapy.
Clinical factors
Twenty-two patients had a ruptured pericallosal aneurysm with SAH, while in one patient SAH was attributed to an aneurysm of medial cerebral artery on the basis of distribution of bleeding and vasospasm on imaging. There were no other symptoms apart from rupture.
Anatomical factors
The mean aneurysm size was 5 mm, ranging from 3 to 10 mm. The majority (68%) was on left pericallosal artery, and approximately half of all aneurysms (52%) had a wide neck. The incorporation of arterial branches in aneurysm neck/sac was present in 76% of patients, callosomarginal artery was more often involved (52% of patients) than pericallosal artery (24%). The lateral orientation of the aneurysm was observed in 2 patients. Anatomical variations included hypoplastic pericallosal artery (PA) in 5 patients (16%) and angulated PA in 6 patients (20%).
Technical factors
Initial complete occlusion of the aneurysm was achieved in 68% of the patients. Stents were implanted in four patients, using Neuroform stents. The following coils were used: GDC, Trufill Orbit, Microvention, Matrix, Micrus. Microcatheters used were Excelsior, Excel, Prowler, Echelon. Statistically significant correlation, with p<0,05, was found between complications and stent implantation. Complications were more often noted in patients with multiple aneurysms, however without statistical significance.
Recanalization was significantly correlated to initial incomplete occlusion, branch incorporation and anatomical variations. It was also more often found in female patients (no male patients experienced aneurysm racanalization), and associated with wide neck of the aneurysm, but with no statistical significance. Initial occlusion was higher in patients treated using Prowler microcatheter and Trufill Orbit coils, however without statistical significance.
Case 1
Figure 1A: Pericallosal artery aneurysm.
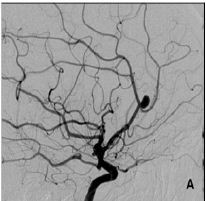
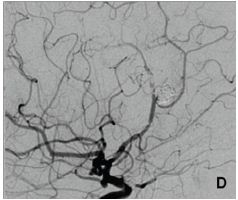
Patient 16 was a 45-year old female with an unruptured right PAA, harboring two other aneurysms, one on the left middle cerebral artery (MCA), and another at the origin of right posterior communicating artery (PComA). PAA and MCA aneurysms were embolised by coils. PAA was initially incompletely occluded in order not to risk the patency of callosomarginal artery, and 6 months later the aneurysm was significantly recanalised, prompting a second treatment (Figure 1A & 1B).
Figure 1B: Recanalistion of the aneurysm 6 months after the coiling procedure.
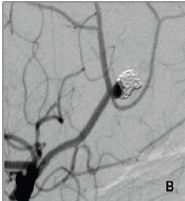
Figure 1C: Small neck remnant et the end of the second procedure. The stent was implanted over the aneurysm neck, and both pericalosal and callosomarginal arteries are patent.
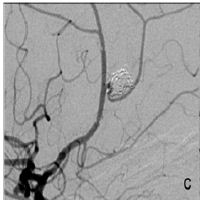
Figure 1D: Thrombus in the distal part of the stent in the pericallosal artery with severely diminished distal flow Figure 1: Digital subtraction angiography.
The aneurysm was then occluded by coils and stent was implanted across the aneurysm neck, in the PA (Figure 1C). The patient received a standard antiplatelet prophylaxis prior to treatment, consisting of 100 mg of acetyl-salicylic acid and 75 mg of clopidogrel daily, during 5 days. She tolerated the procedure well, and was discharged from the hospital after 3 days. On the 5th day she presented with hemiparesis of her left leg, lasting 4 hours at the time of presentation. We found out that she forgot to take her antiplatelet medications after the discharge. Head CT scan was unremarkable, and she was immediatelly transferred to digital angiography, which showed a thrombus in the distal part of the stent with very slow distal flow in PA (Figure 1D). The attempts of intraarterial thrombolysis by Actylise, in a dose of 0.1 mg/kg/hr, and mechanical retrieving of the thrombus were unsuccessful. MRI confirmed acute ischemic lesions in distal right ACA territory, with no hemorrhage (Figure 2A).
Figure 2A: Diffusion weighted magnetic resonance image in axial plane shows multiple acute ischemic foci in the right anterior cerebral artery territory
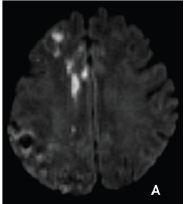
Figure 2B: Brain computed tomography showing multiple small subcortical and basal ganglia hemorrhages, dominantly in the right hemisphere.

Figure 2C: Brain computed tomography showing multiple small subcortical and basal ganglia hemorrhages, dominantly in the right hemisphere.

On the second day she was moderately cognitively impaired with persistant left leg hemiparesis. Her control head CT showed multiple bilateral small hemorrhages subcortically and in the basal ganglia, mostly in the right hemisphere (Figure 2B & 2C). She was transferred to physical therapy and completely recovered during the next three months. The exact etio-pathogenesis of multiple hematomas, outside of the region of initial ischemia, was not clear. The remaining PComA aneurysm is small, with no changes during the xx months of follow up, and the patient was not inclined to further endovascular treatment. The antiplatelet medications were excluded 3 months after the complication. She remains in followup, with no symptoms.
Case 2
Patient 22 was a 28-year old female with asymptomatic unruptured small aneurysms of left PA and right MCA, with a positive history of familial SAH. Her sister was treated in our institution few months before due to a ruptured aneurysm, and the patient was highly motivated to undergo endovascular treatment, although both her aneurysms were small and non-symptomatic. The neck of the PA aneurysm incorporated callosomarginal artery (CMA). During the procedure, the second coil ruptured the aneurysm and at the same time, coil protrusion occluded CMA (Figure 3).
A: Small aneurysm of the left pericallosal artery, incorporating the callosomarginal artery.
B: The rupture of the aneurysm with active bleeding into subarachnoid space, tip of the microcatheter is seen just proximal to the aneurysm neck.
C: Protrusion of coils with the occlusion of callosomarginal artery.
D: Stent implanted over the aneurysm neck with restitution of arterial flow and vasospasm at the A1 segment of anterior cerebral artery and at the distal stent marker. The aneurysm is occluded
Figure 3: Digital subtraction angiography.

We placed the stent (Neuroform EZ, 3 x 15 mm, Stryker Neurovascular) over the neck of the aneurysm, thus opening the CMA and finished the procedure by occluding the aneurysm with one more coil (Figure 3). According to the pre-procedural assessment of morphology and anatomy, it was not expected that the stent placement would be required, so the patient was not on antiplatelet therapy. Immediately after completing the aneurysm coiling, eptifibatide was administered in an iv. bolus of 180 mcg/ kg, followed by an infusion in the dose of 2 mcg/kg/min. Postprocedural course was uneventful, and three days after the initial treatment, she underwent a second session and the remaining right MCA aneurysm was also occluded by coils, with no complications. The patient was discharged without neurological deficit and had no recanisation or neurological symptoms during the 2-year follow up.
This is, to our knowledge, the largest series of endovascularly treated pericallosal artery aneurysms, presenting 31 patients. The aim of our study was to analyze the factors influencing the complication and recanalizaton rate. We hypothesized that anatomical and technical factors were of primary concern regarding aneurysm recanalization, and that complications were related to clinical and anatomical factors. The incidence of PAAs in our study was 3%, and was in accordance with the literature [10,14]. Multiple aneurysms, the presentation with SAH and female predominance were more pronounced compared to previous studies [7,10,14], however the complication and recanalization rates in our patients were not dependent on these factors. In most patients, we found the associated aneurysm on medial cerebral artery (10 patients), possibly due to higher detection rate in anterior circulation, since it was more precisely analyzed because the aneurysm of interest was on anterior circulation.
Clinically manifest complications occurred in 3 of our patients (9.7%), who suffered ischemic stroke in the anterior cerebral artery territory. This is comparable to the 10-18% rate of complications reported in other studies [9-13]. One patient suffered ischemic stroke 3 days after an uneventful stent-assisted coiling procedure because she stopped taking her antiplatelet medications. Another patient had a thrombo-embolic event during coiling, in the phase of an acute SAH, and the attempts of thrombolysis failed. The third patient was treated by parent artery occlusion, in the setting of acute SAH. Reconstructive treatment technique was not successful due to unfavorable anatomy of two wide-neck aneurysms at the left anterior cerebral artery and proximal tortuosity which precluded the use of stent.
Clinically silent intra-procedural rupture of the aneurysm occurred in one patient (3%). This is a significantly lower rate of intra-procedural aneurysm rupture compared to 13-16% reported in previous papers [10,11], which may be due to the continuing advances in newer materials used in the treatment of our patients. It is often difficult to obtain secure access to pericallosal aneurysms, because of their distal location. A micro catheter and micro guide wire have to negotiate tortuous carotid siphon and often the acute angle of the A1 segment origin, necessitating the use of relatively substantial force. This may result in an abrupt entrance into the aneurysm with a risk of rupture, and micro catheter is often unstable in the aneurysm, requiring multiple repositions during the procedure. Furthermore, manipulations in narrow A2 segment of the ACA may result in vasospasm. All these factors make it difficult to choose the right combination of micro catheter and micro guide wire, since the balance between the strength and flexibility is often crucial. Some authors prefer to use a soft micro catheter and guide wire to prevent the risk of aneurysm rupture [10]. In our experience, the risk of rupture may be overestimated, and the use of soft materials may significantly limit the access to aneurysm site and/or the stability of micro catheter in the aneurysm. This may result in lower packing density of the aneurysm and a higher recanalization rate.
The rate of complications significantly positively correlated with the use of stents, since both patients with implanted stents had complications. However, in the case of one of our patients, the stent was successfully used as a salvage procedure, to re-open the parent artery without clinically manifest complications. In another patient, the initial result of stent-assisted coiling was excellent, but the complication occurred after the discharge from the hospital when she terminated her anti platelet medications. So, the stent implantation itself indirectly lead to complication in only one patient. None of the other investigated factors were significantly associated to complication rate.
Aneurysm recanalization was encountered in 29% of the patients and in 16% of them it resulted in the second interventional procedure. This is in line with previous studies, which reported 12- 53% recanalization rate [9-13]. In our patients, it was significantly correlated to initial incomplete occlusion, branch incorporation and anatomical variations.
Incomplete occlusion of the aneurysm at the initial coiling procedure is a well-known factor increasing the risk of recanalization of the aneurysm, confirmed by our results. The continuous pulsatile pressure of blood flow may lead to the compaction of implanted coils and even re-growth of the aneurysm. It is difficult to decide when such a patient requires re-intervention, since it is not clear how much a recanalization increases the risk of rupture. We recommended a second procedure in all the patients who previously suffered SAH, but were naturally more conservative in patients with non-ruptured aneurysms. Two of such patients remained in follow-up, since the recanalized portions of their aneurysms were limited to aneurysm neck and were stable over time. If the aneurysms were shown to have a flow inside the sac, were progressively recanalizing and/or growing, we opted for the re-intervention. Incomplete initial occlusion occurred in 32% of our patients with PAAs, somewhat lower than 42% reported in the literature [10], however this is a relatively high proportion having in mind that all the aneurysms were small, with an average size of 5 mm.
In our experience, the main reason for incomplete aneurysm occlusion at the initial coiling procedure was the incorporation of arterial branches, either PA or CMA, in the aneurysm neck. It occurred in the majority of our patients (76%). Although there are recent reports with good results using Y-stent technique [15], we felt that in such small caliber arteries, the reconstruction techniques as balloon remodeling and stenting increase the risk of intimal injury and perforation of the artery. We did not use balloons in any of our patients with PAAs, and used stents in two described patients. This resulted in a more conservative coiling technique in order to preserve the patency of arterial branches and in turn did not allow for complete embolizations.
Anatomical variations and aneurysm configuration were also associated with higher recanalization rate. We noted three typical variations: hypoplastic and angulated pericallosal artery and laterally oriented aneurysm. These factors lead to difficulties in the access of the aneurysm with a less stable position of the micro catheter. One of the patients with a wide-neck, laterally oriented PCA and another callosomarginal aneurysm who was treated by the occlusion of proximal aneurysm and the parent artery, experienced stroke.
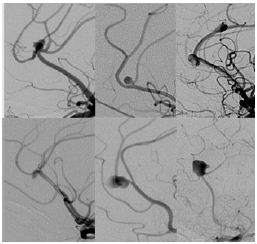
Despite the results in aneurysms at other sites, the size of the PAAs was not associated to recanalization or incomplete coiling, probably because all these aneurysms were small. The configuration of PAAs has been mentioned as an important factor in endovascular treatment of this group of patients [10]. However, in our experience, morphology, orientation and anatomy was highly variable, not allowing the classification of patients based on these characteristics. Therefore it was not possible to define risk factors for complications and/or recanalization depending on morphology and anatomy (Figure 4).
Figure 4: Digital subtraction angiography showing highly variable anatomy of pericallosal artery aneurysms.
In conclusion, endovascular treatment of pericallosal aneurysms is demanding due to small size of aneurysms and caliber of parent artery, and relatively broad aneurysm neck which is in close proximity to the origin of arterial branches. We investigated numerous clinical, technical and morphological factors to determine their impact on recanalization and complication rate of the treatment. The rate of complications and recanalization proved to be higher compared to other aneurysm sites. Pericallosal aneurysms in our study were however very morphologically and anatomically heterogeneous, making our sample size insufficient to show the impact of these factors on complications. The patients with relatively large aneurysms and those which incorporated branches and the angulation of artery were shown to be more prone to recanalization.


