Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
*Khaldoun El Abed
Received: June 23, 2017; Published: June 28, 2017
Corresponding author: Khaldoun El Abed, Department of Consultant Orthopaedic Spine Surgeon, AlHabib Medical Group, Saudi Arabia
DOI: 10.26717/BJSTR.2017.01.000153
Image-guided technology has transformed spinal surgical interventions, in this review article the author attempts to understand the interactions with the most commonly used 3D system with the intraoperative cone beam CT, and develop a smooth workflow surgical plan. The system facilitates complex surgeries, minimizes radiation exposure to OR staff, and has advantages for minimally invasive surgeries. Aiming for safer surgeries, and understanding that navigation error is an interaction between technology and human factors.
Keywords: Thoracolumbar; Cervical; Pedicle screw; Image-guidance; Spine navigation
Abbreviations: CT: Computed Tomography
The technology used to acquire imaging for intra operative surgical navigation, has evolved from the discovery of X-rays in the late 19th century to the highly sophisticated intra operative Computed Tomography (CT) based navigation tools used today. Navigation has emerged as one of the most reliable representative of technology; as it continues to transform surgical interventions into safer and less invasive procedures [1]. The range of available technologies includes C-arm fluoroscopy, preoperative CT based navigation, 2D fluoroscopy based navigation, cone beam CT based navigation, and intraoperative CT based navigation. Aside from fluoroscopy, these imaging modalities implement the basic steps of image acquisition, registration to patient anatomy, processing, and navigation [2], the tracking systems are generally classified into magnetic, acoustic, laser; and infrared, the latter is the most commonly used and will be discussed in our review.
Spinal instrumentation has made significant advances in the last two decades, with transpedicular constructs now widely used in spinal fixation. Pedicle screw constructs are routinely used in thoracolumbar-instrumented fusions, and in recent years, the cervical spine as well. Three-column fixations with pedicle screws provide the most rigid form of posterior stabilization [3]. Precision in pedicle screw placement is of utmost importance in any spinal fixation procedure, however, misplacement rates have been reported to range from 5% to 41% in lumbar spine, and from 3% to 55% in the thoracic spine when using conventional techniques [4], with as many as 7% of these misplaced screws resulting in neurological injuries [5]. The most commonly used forms of imageguided navigation in spine surgery presently include 2D images, in which a fluoroscope or plain radiography is used, and 3D navigation, making use of cone-beam CT or CT scans. The 3D systems provide projections of the operative field and instruments with imaging in 3 axes [6].
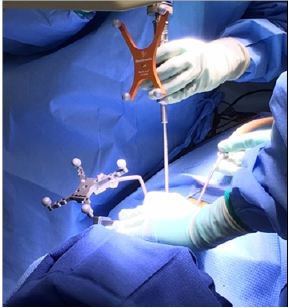
Figure 1: Commonly used method is by optical tracking using cameras that project and detect reflected infrared light from reflecting spheres.
The principle goal is to track surgical instruments and anatomy in the operative field relative to a registered reference point [7], the most commonly used method is by optical tracking using cameras that project and detect reflected infrared light from reflecting spheres (Figure 1) or light-emitting diodes. This technology enables the surgeon to navigate the patient’s spine anatomy using a visual image that shows the position of tracked instruments relative to the surgical field [8]. The 3D fluoroscopic image guidance systems demonstrated an accuracy rate of pedicle screw insertion of 95.5%, and the accuracy rates when 3D was compared to with 2D fluoroscopic navigation were also consistently higher throughout all individual spine levels [9].
Various methods used to register the surgical space with image guidance have included material fiducials, anatomical fiducials, and surface-based registration techniques. The use of material fiducials in spine surgery had drawbacks because it required the attachment of fiducials prior to surgery, resulting in inaccuracy when used with spinal navigation, and is not currently used [10]. Anatomical fiducials required certain anatomically accessible targets to be exactly identified by the surgeon, and then these points in the intraoperative space were matched to CT-based imaging. However, this process, known as paired-point matching, was the least accurate because the surgeon had to identify the exact anatomical location, which remains a difficult task [11]. A variant of the anatomical registration includes surface-based point-pairing techniques. Once registration has occurred, various instruments with reference markers can be appropriately localized in the surgical field, and the tool tips and projections in the axial, coronal, and sagittal planes are computed and projected for the surgeon. A number of possible sources from which errors arise have been documented in early publications on spinal navigation and imageguided registration accuracy [12].
The introduction of cone-beam CT enabled multiple fluoroscopic image acquisition by a device that rotated isocentrically around the patient. The images are reconstructed into a cone-beam CT scan that can be used for navigation once it is transferred to an image-guided system. As the reference arc is tracked with the patient imaging, the computer-generated 3D image of the patient’s operative field is already registered and ready for use with navigation. Advantages with the use of this technology include the ability to image multiple levels in a single sequence, imaging accuracy in patients who had undergone prior spine surgeries at the same levels, decreased radiation exposure to the operating room (OR) staff, improved accuracy because the patient’s anatomy is registered in the surgical position, and portability of the system so it can be easily transported between Operating rooms [13]. The author; an Orthopaedic Spine Surgeon, familiar with setting up the Stealth Navigation combined with the O-arm at different sites in his career, attempting to understand the surgical interactions, and develop a smooth workflow surgical plan (Table 1). For spine surgery, the learning curve for adopting image-guided technology has been reported [14,15], the components of the learning curve include the ability to direct instruments based on imaging visualized on a screen, the ability to replicate in-line manoeuvres while placing instrumentation, as well as adopting and developing proper technique while using image-guided technology [13].
Table 1: Develop a smooth workflow surgical plan.

The Jackson table enables greater deformity correction and correction of alignment along the entire neuraxis, making it ideal for complex spinal fusions. The design of the O-arm allows it to work ideally with the Jackson table, which does not have a base obstructing movement along the long axis of the patient and table. The Jackson table enables the O-arm to be positioned along any level of the spinal axis. The table is well designed for imaging purposes, with its core structure such that the table has minimal radiodense metal resulting in minimal radiographic artefact (Figure 2). Preoperative patient factors include the potential difficulty of performing adequate imaging on obese and morbidly obese patients. The increased soft tissue in patients with morbid obesity may create difficulty with positioning, beam penetration, and the ability to manoeuvre imaging devices around the patients. This results in poorer quality images that can make the registration process inaccurate, as well making the images difficult to use during surgery [16]. Movements during respiration and image acquisition can cause significant changes and inaccuracies with registration. The anesthesiologist has to specifically hold the patient’s respiration during this step, minimizing errors secondary to motion artifact. Image acquisition is also performed after the dissection, and afterward the deep retractors are left in situ, especially for mobile cervical spine segments. The surgeon should avoid changing the position of the table (Trendelenburg position or the reverse) and attempt to avoid any potential movements with instruments that may cause distortion of anatomy, such as drilling and tapping all holes prior to instrumentation.
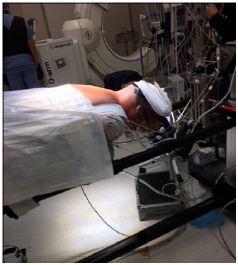
Figure 2: Has minimal radiodense metal resulting in minimal radiographic artifact.
There is potential inaccuracy with increasing distance of screw placement from the reference arc, and increased the duration of surgery [17], with inaccuracy of 3 mm in 7% of the patients when surgery was 3 levels away from the reference arc, and inaccuracy of 3 mm in 17% about 1 hour into surgery. Scheufler et al. performed pedicle screw instrumentation using a single-registration sequence in 32 (91.4%) of their 35 patients, with as many as 12 vertebrae instrumented after a single registration sequence. They identified a statistically insignificant increase in misplaced screws by about 2 mm at distances 10 segments between the instrumented segment and reference arc [18]. We read with interest what Holy et al [19] demonstrated that navigation error is an interaction between technology and human factors, remaining the same independent of registration techniques.
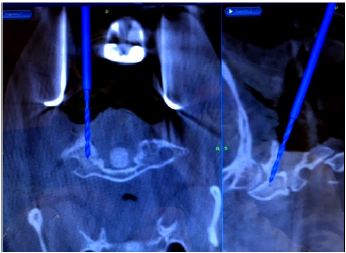
Figure 3: The bone anatomy and placement of retractors to minimize movement after image acquisition and registration.
For posterior cervical spine surgery (Occipitocervical fusion), the reference arc is recommended to be placed on the Mayfield head holder [20], taking into consideration the trajectory of upper cervical screws, to minimize intersegment movement in the upper cervical spine, imaging should be performed following dissection up to the bone anatomy and placement of retractors to minimize movement after image acquisition and registration (Figure 3).
The size and depth of the bore, patient positioning, and orientation are limiting factors for the use of fluoroscopic systems as well as cone-beam CT–based systems. In these cases, it is ideal to perform fluoroscopy-based imaging, utilizing surgical landmarks and imaging landmarks as guides for the procedure. Ideally, use of radiolucent retractors avoids imaging artifacts, but if these are not available, standard retractors have to be removed to obtain clear images without distortion. The spine surgeon should keep in mind that navigation is useful for bone anatomy and not useful in delineating the adjacent vascular anatomy or soft-tissue structures.
Recently there has been increasing interest and use of lateral and oblique approaches to the lumbar spine to perform interbody fusions in patients with spinal deformity, spondylosis adjacent to a previous fusion, or degenerative disc disease. Spinal navigation systems have been adapted to these procedures and the dissection, discectomy, and implant tools can be currently paired to navigation to help increase the safety and efficiency of these procedures. Navigation simplifies patient positioning, enabling patients to be easily placed laterally on a Wilson frame, on top of a flat table rather than manoeuvring the operative table to perfectly align with anteroposterior and lateral fluoroscopy. The navigated dissection and discectomy tools help to confirm appropriate passage through the tissues and disc space. Preoperative planning with proper placement of the reference arc is crucial. The reference arc must be placed in a position that can be seen by the camera, close enough to the operative field to maintain accuracy, and out of the line of sight to the operative tools. Placing a percutaneous reference arc into the posterior iliac crest serves better than placement into the lateral iliac crest. The lateral crest site may obscure visualization of the arc, and because of the proximity to the surgical site, interferes with the operative tools as they are placed into the disc space. Operating Room Set up (Figure 4).
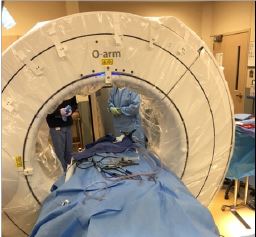
Figure 4: Operating Room Set up.
The type of spinal fusion case and surgeon preferences are entered into the image-guided system. The ability to view the reference arc, and the image-guided instruments within this surgical field, requires a linear trajectory between the infrared camera, the reference arc, and the instruments. This direct line of sight requires appropriate placement of the infrared camera and the reference arc. Incorrect reference arc positioning, as well as placement of objects that block the line of sight between the infrared camera and the reference arc, will result in line-of-sight issues with a resultant inability to navigate. Subsequently, time and frustration will be added to the case. If the line of sight is clear between the camera, the reference arc, and the instruments, and the image-guided system still does not visualize the image-guided instruments or reference arc, then the most likely cause is blood or other debris on the reflective spheres of the reference arc and/ or image-guided instruments. In this scenario, the spheres should be cleaned with a wet towel or gauze pad and then dried. Bending of the instruments after registration can be problematic because remote instrument tracking relies on the stiffness and no deformity of surgical instruments. Instruments that are optically tracked and thin such as K-wires or drill bits could bend and result in inaccurate navigation [21].
Figure 5: Sterile draping for the O-arm is cumbersome.
It is essential to firmly fixate the reference arc to the bone anatomy in lumbar and thoracic cases to ensure that the arc does not move relative to the patient’s spine after cone-beam CT registration. It is important that the spikes on the clamp of the reference arc penetrate the cortical bone of the spinous process to prevent the clamp sliding on the bone. Interspinous placement of the reference arc has to be avoided. We gently pull on the reference arc after it is fixated, and before cone-beam CT registration, to confirm that fixation is secure. It is also important to apply counter traction on the reference arc stem when screwing the clamp onto the spinous process to prevent fracture of the spinous process. After cone-beam CT registration, it is essential that the surgeon and assistant do not bump the reference arc, which could move the arc relative to the patient’s spine and result in navigation inaccuracy. Additionally, suction tubing and wires for the cauterization instruments should be positioned so that they do not contact or put traction on the arc when being used. Sterile draping for the O-arm is cumbersome (Figure 5), and at times getting caught between the shields, in most cases, to avoid the O-arm draping, surgical split-sheets drapes are used to circumferentially enclose the patient, keeping the reference arc just above the drapes (Figure 6), following imaging, these drapes are removed and procedure continued.
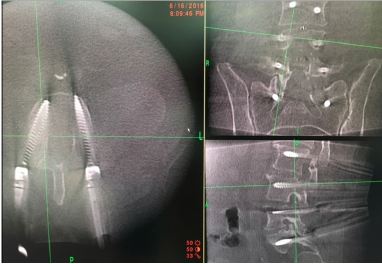
Figure 6: keeping the reference arc just above the drapes.
Fluoroscopy can increase the radiation exposure to the surgeon by 10- to 12-fold in comparison with no spinal procedures, and more so with complex multisegmental fusions, deformity corrections, and reoperations on the spine [22]. With the use of low-dose helical CT techniques, a resulting 20-fold decrease has been noted in patients’ radiation doses compared with standard CT [23]. Nottmeier et al [24] demonstrated the absence of any radiation exposure to OR personnel using cone beam CT–guided imaging systems with absence of any radiation scatter if the surgeon stood more than 10 feet away from the system and behind a lead screen. It is important to keep in mind that the surgeon will perform a large number of these procedures in a year, whereas the patient may not require further imaging in the postoperative period once intraoperative imaging confirms appropriate implant insertion following surgery.
Various studies have shown that spinal navigation can be used without increasing OR time, and possibly with even shorter times compared with conventional surgery [25]. Costa et al [26] evaluated the cost-effectiveness of intraoperative spinal navigation with preoperative planned navigation systems. In their study of hospital costs in patients with spondylolisthesis, they concluded that the two technologies were similar in cost. Despite these data, there may be a trend to suggest a cost advantage of using image-guided navigation in high-volume centers that specialize in more complex cases and perform more than 150 cases a year. The initial cost of the navigation system is high, and cost-effectiveness depends on different medical fees according to each country.
The users of navigation cited increasing accuracy, facilitating complex surgery, minimizing radiation exposure, performing a high volume of surgeries, and its use for minimally invasive surgeries as advantages. With an increasing number of intraoperative imaging and navigation options being made available to surgeons, integrating effective training and shortening the learning curve are essential to making this technique cost-effective and safe. However, if the surgical team is aware and takes into account the above interactions, image-guided systems enable safe and accurate placement of spinal instrumentation in both routine and challenging situations.


