Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Giulia Bora1, *Flavio Colaut2, Gianni Segato3, Luisa Delsedime4 and Alberto Oliaro1
Received: June 14, 2017; Published: June 26, 2017
Corresponding author: Flavio Colaut, Department of General Surgery, City Hospital Montebelluna, Thoracic City Hospital, via Montegrappa 1, 31044 Montebelluna (Treviso), Italy
DOI: 10.26717/BJSTR.2017.01.000150
Solitary fibrous tumor of the pleura is a rare neoplasm. In Literature up to 800 cases [1-3] have been reported, and these numbers show its rarity, despite of mesotheliomas, the most pleural tumors represented. Males and females are equal distributed and the same is true for age. No correlation with exposure to asbestos, tobacco or others environmental agents, were found for its development. Solitary fibrous tumor of the pleura occurs as localized neoplasms of the pleura and was initially classified as “localized mesothelioma”. Recently, with the aid of the electronic microscope and immunohistochemistry, has been possible emphasize that their origin is mesenchymal and not mesothelial, so the term “localized mesothelioma” has been replaced with “solitary fibrous pleural tumor” STFP [4]. In the past STFP were described only in the pleura but recently it has been found located also in other sites [5,6] such as abdomen, liver, peritoneum, retroperitoneal spaces, meninges, orbit, thyroid, salivary glands and soft tissues including the breast [7-10]. The STFP can be associated to other synchronous or metacron neoplasms like prostate, lung, breast, endometrial carcinoma and thyroid. Although most of STFP are benign neoplasms, a part of these could have a malignant behavior. The clinical behavior is unpredictable and probably is due to their histological and morphological features. STFP may remain silent for many years before turning into malignant form [11].
Often SFTPs are accidentally discovered by radiological investigation like X-Ray [12,13]. Most patients with SFTP become symptomatic when these tumors reach large size [2,10,14]. About 54-67% of patients with benign SFTP are symptomatic, while 75% of cases of malignant STFP, show symptoms [15]. These symptoms are cough, chest pain and dyspnoea. If there is obstruction of the airway, also hemoptysis and pneumonia [2,10] could be seen. Paraneoplastic syndromes, represented by digital hypocratism and Pierre-Marie-Bamberg syndrome, are observed in 10-20% of cases and specially with large size SFTPs. Symptoms usually vanish after the neoplasm has been removed, but may reappear in case of recurrence [10,16,17]. In less than 5% of patients with SFTP an increase of insulin-like factor II type occur and this causes refractory to therapy hypoglycaemia (Doege-Potter syndrome) [10,18,19]. The incidence of Doege-Potter syndrome in SFPT is similar in both sexes and there no differences in both benign and malignant forms.
Some patients may also present gynecomastia or galactorrhoea [1]. Sometimes large size SFTPs may appear with an unusual clinical presentation, as the two cases reported by Santambrogio et al. [20] and Shaker et al. [21]. The first described a patient with large size SFTP manifested with syncope episodes of coughing. The second author reported the case of a woman with lower limbs edema and dyspnea caused by a bulky SFTP compression to the right atrium and the inferior vena cava.
England [10] suggested SFTPs originate from sub-mesothelial connective tissue. Histologically SFTPs occur such as low-grade neoplasms with variable cellularity. The cancer cells present oval or fusiform shape with oval nuclei and chromatin is well distributed. Electronic microscopy has identified at least 3 pathological patterns: “subverted order” is the most common. In this type the cells and the surrounding collagen do not exhibit any architecture. The second most common pattern is “hemangiopericytoma-like”, in which dense anastomoses are highlighted among blood vessels. The third one is the least frequent and refers to angiofibroma-like, fibrosarcoma-like patterns and synovialsarcoma-like.
Histologic differential diagnosis is difficult and includes spindle-cell melanoma, sarcomatoid mesothelioma and soft tissue sarcomas. Recently immunohistochemistry has revealed to be extremely usefu for differential diagnosis. Perrot et al. [2] summarized the most important immunohistochemical characteristics: in SFPTs vimentin is positive and keratin negative. CD34 is + in most benign SFTPs as well in malignant one, while it remainsnegativeinmostothertumorsofthelung. Lately, a cytogenetic procedure has also contributed: SFTP is often associated with trisomy 8 and trisomy 21 and this is helpful for diagnose and differentiate fibrous pleural tumor from mesothelioma and other sarcomas. Genomic hybridization has been shown that these chromosomal abnormalities are more frequent in SFTPs larger than 10 cm and this correlation may suggest that gene mutations can promote the growth of neoplasia. Also SFTP’s maliciousness prediction is not easy. England et al. [10] defined malignancy criteria (Table 1).
Table 1: Malignancy criteria by England et al. [10].
Perrot et al [11] according to the revision of the literature divided SFTP in four stages, correlating histologic features with clinic presentation:
Stage 0: SFTP pedunculated without signs of malignity
Stage 1: SFTP sessile without signs of malignancy
Stage 2: SFTP pedunculated with histologic signs of malignancy
Stage 3: SFTP sessile with signs of malignancy
Stage 4: Multiple and metastatic SFTP
The malignancy criteria used in this classification include the presence of hypercellularity, atypia, cellular pleomorphism, increased number of mitosis, high number of mitosis per field, necrosis and invasion stromal / vascular.
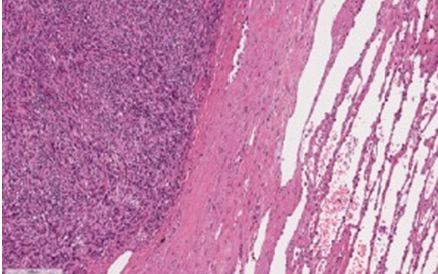
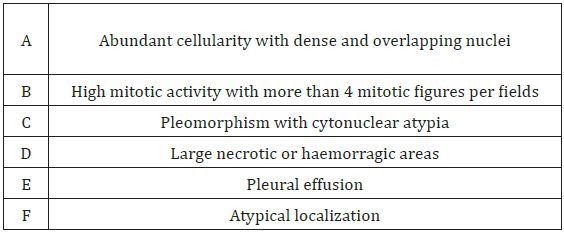
Figure 1: Hematoxylin-eosin stain 100x.
Macroscopic description: 5 gray-whitish rounded suture shrubs: the first cm 12x7.2x7 size, the second cm 13x9x8 size, the third cm 10x9x7 size, the fourth cm 5x5x1.5 size and the fifth one cm 2.5x1.8x1 size. All fragments include irregular surface, with fibrous pseudocapsula, in appearance lobulated with mucoid areas on the surface of the section; multiple nodules are surrounded by unhindered lung parenchyma rhymes. The nodes macroscopically described are constituted by proliferation of fused cells, circumscribed by fibrous pseudocapsules.
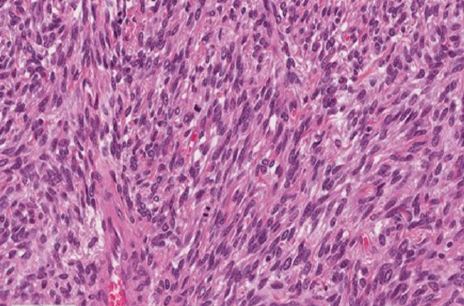
Figure 2: Hematoxylin eosin stain 400x.
Macroscopic Description: these neoplastic cells have bulky, ovoid, monomorphic nuclei; mitotic 14/10 HPFs (two evident mitosis in photo 2); Focal areas of necrosis are present. There are also areas of sclerosis and stromal and perivascular healing.
Figure 3: Hematoxylin-Eosin stain 200x.
Macroscopic Description: Proliferation cells were found to be intensely positive to immunohistochemical reaction for CD34 (see photo 3, 200x) and for CD99 (+).
By gentle courtesy of Luisa Delsedime, Pathology, University of Turin
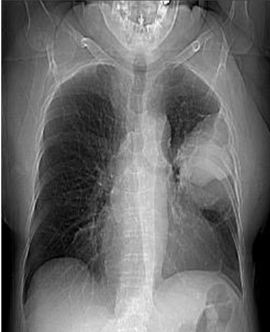
Figure 4: Rx chest.
Chest X-ray shows a well-defined mass that is localized typically in the surface of the lung. SFTP is often located in the middle and lower lobes [22] (Figure 4).
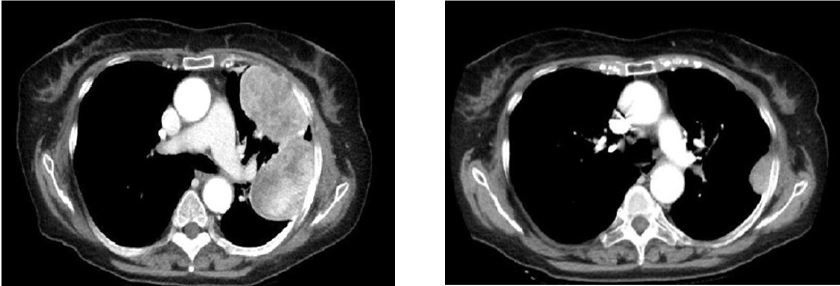
Figure 5: CT scan. Pleural nodulations on the left side.
SFPT CT scan typically demonstrates homogeneous nodulations, well defined on the pleural surface. In some cases it results difficult to distinguish SFTP from interlobar masses [23]. In some SFTP calcifications can also be observed, regardless of the benignity or malignancy of the lesion [10,11] and it can be difficult to differentiated them from a bronchial carcinoid [24]. With TC Scan is not possible differentiate a benign lesion from malignant one and even the dimensions do not correlate with the behaviour of the neoplasm [25,26] (Figure 5).
Magnetic resonance imaging (RM) has a limited use in pleural diseases. RM investigation is better to TC for delineate morphology and the relationship large-size SFTPs with adjoining mediastinal structures, like great vessels and with the diaphragm [11,27-29].
Angiography is an important diagnostic tool. It allows localizing the SFTP’s vascular peduncle [30]. The demonstration of arterial supply from the frenic artery, intercostal artery or from the internal mammary vessels, can be very helpful to determinate the extrapulmonary origin of large-size SFTPs.
The indication to perform an ultrasound chest scan for diagnoses SFTP or its evaluation isn’t shared by many authors, except for a biopsy trans-thoracic for typing [31].
Recently has been documented that FDG-PET can be used to diagnose and for follow up of treated patients. Cardillo et al. [32]. In their study confirmed the high negative predictive value of PET for assessment of the malignancy behaviour of the lesions. In the case of multiple SFTPs and a high metabolic rate highlighted by FDGPET, a malignant nature must be suspected [33].
The main differential diagnosis of malicious SFTP includes mesothelioma, neurogenic sarcoma, synovial sarcoma, haemangiopericitoma, fibrosarcoma and malignant fibrous hystyocytoma [34-36]. A great help for diagnosis comes from immunohystochemical analysis.
The best treatment for SFTP is radical and complete surgical excision of the neoplasm because of the high rate of recurrences [37]. Pedunculated tumors can be resected with a wedge resection, but sometimes even a lobectomy is necessary, partial pleurectomy or even a resection in block with part of chest wall. If the neoplasm is adherent to the parietal pleura, dissection must be used by extrapleuric via [16,38].
Regarding surgery access, it can be used postero-lateral toracotomy, anterior thoracotomy as well as the approach by Video-Assisted Thoracoscopic approach, according to the size of the tumor and its location.
Currently, adjuvant chemotherapy is used after surgery resection and it’s recommended in malignant SFTP, especially in recurrent forms.
In SFTP neo-adjuvant chemotherapy is limited due to difficulty of obtaining a pre-operative histologic diagnosis [39].
Prognosis of SFTP is generally good (88%), but approximately 12% of the cases evolves in spread of intrathoracic or non-resectable recurrence. The relapse occurs up to 17 years after resection and is usually located in the same emithorax [35]. Intrathoracic recurrence can be lethal for mediastinum compression and for the inferior vena cava obstruction [18]. Metastases spread occur by hematogen via and generally they are localized to liver, central nervous system, spleen, peritoneum, adrenal gland, gastrointestinal tract, kidney and bone.
After resection of SFTP malignant sessile the risk of relapse is higher. Most SFTP relapses of malignant sessile occur within two years from resection and approximately about 50% of the relapses is the cause of death. Therefore, is recommended check with Rx or TC scan every six months during the first 2 years and then once a year. In case of recurrence is indicated surgical treatment [39]. The most important predictors are morphologic and histological indicators [4].
De Perrot [11] proposed a SFTP’s classification based on their characteristics and prognosis:
A. recurrence of 2% in pedunculate benign tumors
B. recurrence of 8% in sessile benign tumors
C. recurrence of 14% pedunculated malignant tumors
D. recurrence of 63% and mortality of 30% within the first 2 years in malignant sessile FSTP
E. pleural effusion
F. atypical localization
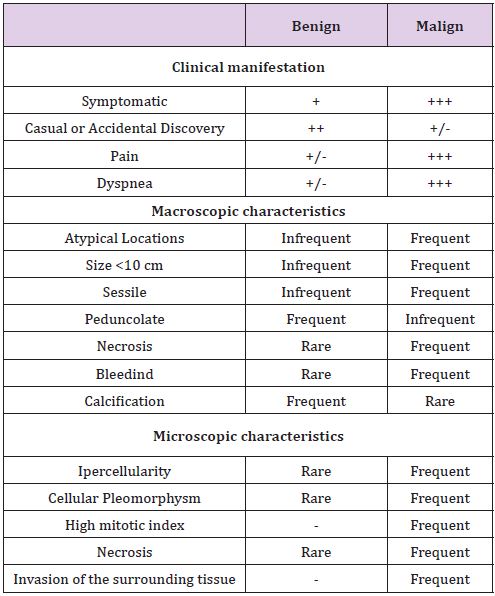
However more than dimension histological features are important [11] and the most important indicator of clinic outcome is the complete recability of the tumor at the first presentation (Table 2).
Table 2: SFTP clinical manifestations related with frequency of symptoms.
SFTP is a rare disease with uncertain behaviour, curable in most cases. From a biological point of view, we need more knowledge to optimize the clinical outcome. Immunohistochemistry is very useful. Indeed, in addition to the reaction with CD34, the recent introduction of protein STAT36 seems to allow a better and more accurate diagnosis. The best treatment remains the surgical resection, especially earlier and radical at the presentation. Because of the uncertain behavior after surgery, a long follow-up is mandatory and in case of recurrences it is necessary to consider the option of a re-surgical approach.


