Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
*Alcaraz Rubio Jesús
Received: June 18, 2017; Published: June 26, 2017
Corresponding author: Alcaraz Rubio Jesús, Coordinator of the unit of Hematology in Unión Murciana de Hospitales, Murcia, Spain and the Regenerative therapy unit of Hospital la Milagrosa, Madrid, Spain
DOI: 10.26717/BJSTR.2017.01.000149
Keywords: Blood Products; Haemovigilance; Transfusion; Donation; Hemaopoietic Growth Factors
The blood transfusion is an essential part of modern medical care. Used properly can save lives. Throughout the world, the centers that make blood products strive to provide safe of them, since the process of the transfusion could be without risks. At this point is essential the rational use of the same, imposing the need to balance the benefits and potential side effects that could produce like allergic and hemolytic reactions, metabolic processes, effects on cellular immunomodulation, lung damage or transmission of infectious diseases (HIV, HBV and HCV, among others).
According to the data of the American Society of Blood Banks (AABB), approximately 85 million units of red blood cells administered annually, making the transfusion such as 8th key of all interventions capable of saving lives in centers that perform surgery (35% of the total needs of blood products), emergency obstetric care (25% of the transfusion requirements), organs and tissues transplants (20% of the requirements of blood transfusion), traffic accidents (10% of the total blood products administered, oncological patients (6% of the transfusion requirements) and chronic diseases (4% of the needs of blood products) (Figure 1).
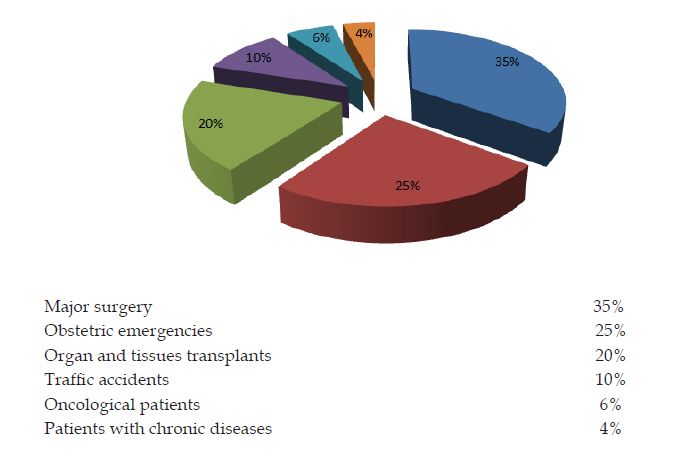
Figure 1: Transfusion Requirements in Hospital Units.
The donation, source of different types of blood products, must be voluntary, altruistic and non-remunerated, running as the safest in terms of minimizing the possibility of transmission of infectious diseases, thus ensuring the quality, availability and accessibility of blood transfusion. This process facing the individual and where a series of internal and external elements that interact to influence risk factors for presenting an adverse reaction to donation, understanding this as any unexpected event that appears at the time of the donation and that puts at risk the integrity, stability and/or the health of the donor, by causing disability and/or disease.
On the other hand the improvement and greater accessibility of laboratory tests and their indiscriminate use, constitute the basis of the decision to transfuse, resulting in an inappropriate use of the blood products. In this context, it is essential to the establishment of clinical protocols, supported in the relevant complementary analytical tests mandatory for the staff involved in the transfusion act at a given time in order to optimize the use of this valuable resource. We always have to transfuse the patient, not to the analytical”.
For all of the above at the beginning of the 90 years in England arose what is known as the Haemovigilance, with the objective of improving and ensure the safety in both patients and donors; and which consisted in the establishment of a national system of registration, definition, classification and monitoring of those adverse reactions associated with transfusion that may occur in both the donor and the recipient.
Finally the so-called: hematopoietic growth factors” that are protein substances which could regulate hematopoiesis to interact specifically with receivers located in membrane of progenitor cells from bone marrow and among its functions is to stimulate the endogenous production of blood cells as an important alternative, with the aim of reducing the homologous transfusions. Administered in patients in whom it is expected a hemorrhage, would be the following benefits: Shorter hospital stay, less postoperative infection, absence of complications, better prognosis of patients by avoiding the use of allogeneic blood. For this purpose since 1997 is authorized the use of recombinant human erythropoietin (rHuEPO), in patients exposed to surgical procedures, such as complementary therapy in programs of an autologous donation or as a single treatment. The use of rHuEPO, in these patients with high risk of bleeding, aims to reduce or eliminate the allogeneic blood transfusion, which has proven to be effective.


