Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Goutam Roy Chowdhury1, Sangita Agarwal2 and Abhijit Mitra*3
Received: June 09, 2017; Published: June 16, 2017
Corresponding author: Abhijit Mitra, Department of marine science, University of Calcutta, 35 B.C. Road, Kolkata 700091, India
DOI: 10.26717/BJSTR.2017.01.000138
Astaxanthin, a naturally occurring carotenoid pigment possessing strong antioxidant property has been detected to play a vital role in the protection against lipid peroxidation and oxidative damage of LDL cholesterol, cell membrane, cells and tissues. It is available from several biological sources particularly the microalgal species Haematococcus pluvialis, but the salt tolerant mangrove vegetation present in the deltaic lobe of Indian Sundarbans has been documented as one of the most potential sources of astaxanthin. This paper documents the accumulation of astaxanthin in six species of mangroves (Avicennia officinalis, Avicennia alba, Avicennia marina, Sonneratia apetala, Aegiceros corniculatum and Bruguiera gymnorrhiza) at ten different stations having different environmental conditions in the Hooghly-Matla estuarine complex of Indian Sundarbans. Although these six species share the same brackish water media, but significant variation in the leaf astaxanthin level confirms the concept of species-specificity and effects of various physico-chemical variables on mangrove astaxanthin.
Astaxanthin is a member of carotenoid family. These molecules are associated with many of the colours that are seen in leaves, flowers and fruits. This pigment is ubiquitous in nature, especially in the marine environment and is probably best known for eliciting the pinkish-red hue in the flesh of salmonoids, shrimp, lobsters and crayfish. In marine ecosystem, astaxanthin is biosynthesized in the food chain by microalgae or phytoplankton, as the primary production level. Microalgae are consumed by zooplankton, insects or crustaceans that accumulate this secondary carotenoid and which, in turn, are ingested by larger animals that will then take on a pinkish-red colour. In nature, a typical xanthophylls-producing unicellular microalgae is Haematococcus pluvialis, well known for its massive accumulation of ketocarotenoids, mainly, astaxanthin upto 4% of its dry mass and its acyl esters, in response to various stress conditions, e.g. nutrient deprivation or high irradiation.
Also, the yeast Phaffia rhodozyma has been widely used for astaxanthin production in fed-batch fermentation processes using low cost materials as substrates An et al. [1], Chociai et al. [2], Vazquez et al.[3]. Because of antioxidative properties and the increasing amounts of astaxanthin needed as a supplement in the aquaculture of salmonoids and other seafood, there is growing interest in the biotechnological production of astaxanthin. The present paper is the outcome of a research endeavour carried out during postmonsoon season of 2015 to screen the mangrove vegetation for astaxanthin in the Indian Sundarbans region. This deltaic lobe is situated at the apex of the Bay of Bengal and has been designated as World Heritage Site for its marvelous genetic diversity with respect to mangroves and its associated flora and fauna [4-6]. Mangroves are special types of vegetation, which are usually restricted in the coastal areas and are characterized with the presence of special features like presence of pneumatophores, stilt roots, prop roots and salt glands in their leaves. There are 34 species of true mangroves in the present geographical locale with several ecological, pharmaceutical and economic utilities Mitra [4], but only six dominant species at ten different stations were selected for the present study.
The entire network of the present programme (conducted during February, 2015) consists of the sampling of the leaves of selected mangrove species during the low tide period from ten different stations, namely, Canning, Gosaba, Bali Island, Chotomollakhali Island, Jharkhali, Sagar Lighthouse, Kachuberia, Chemaguri, Harinbari and Henry’s Island in the central and western sectors of Indian Sundarbans. Salinity, pH, temperature, dissolved oxygen and nutrient load of the ambient water were analysed simultaneously to pinpoint the hydrological parameters to which the vegetation are exposed in natural condition. The collected leaves were thoroughly washed with ambient water followed with deionized water and oven dried at 1100C overnight. The extraction of carotenoid were separately carried out for each species through Dimethyl sulphoxide (DMSO) with maximum absorbancy (approx. 471-477 nm) against an acetone blank on the spectrophotometer and finally converted to astaxanthin percent as per the expression:
Carotenoid (mg) extracted= Abs. Max. / 250 × 25ml acetone × dilution
Percent Astaxanthin = Carotenoids (mg) extracted / sample wt (mg) × 80
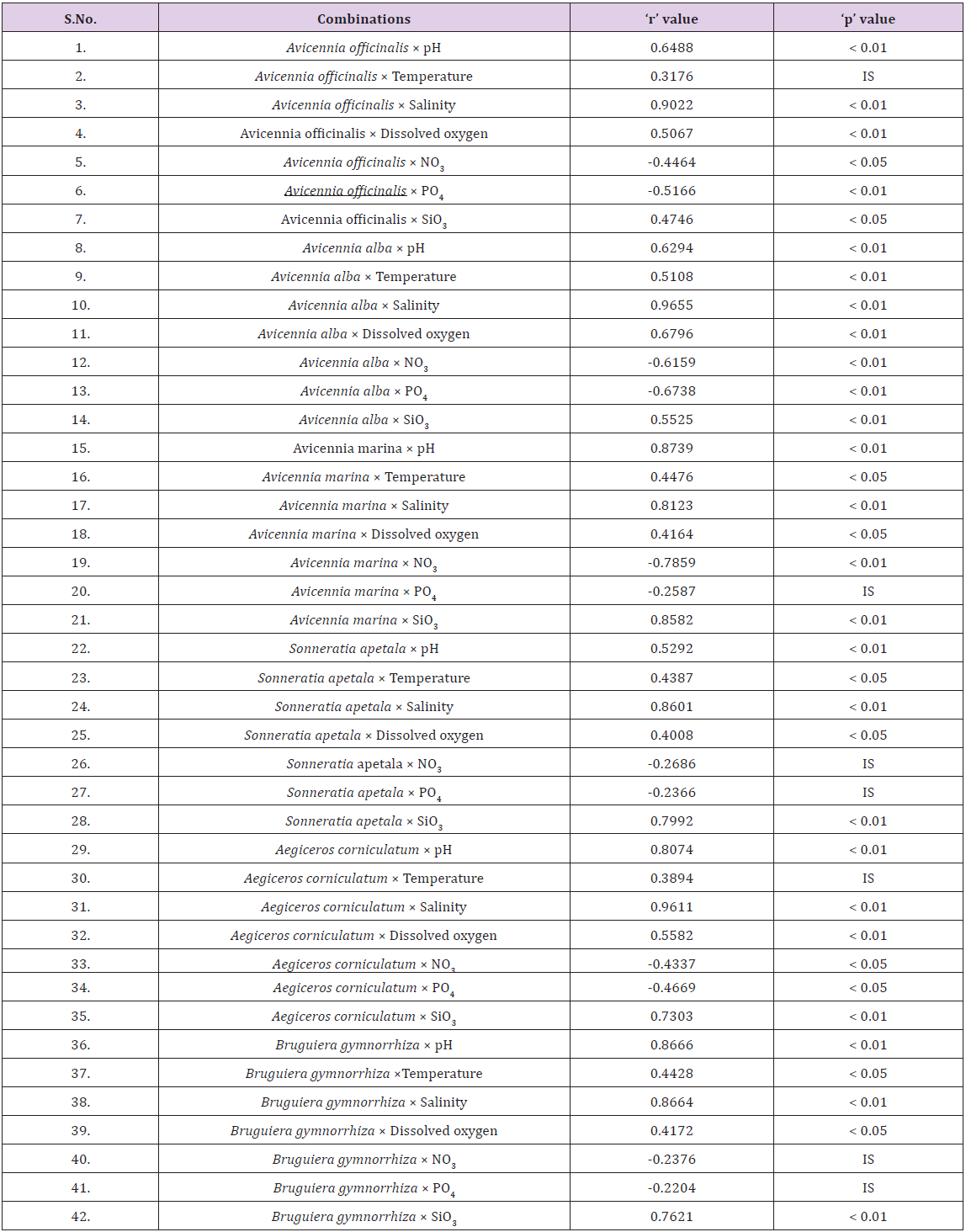
The Indian Sundarbans in the lower Gangetic delta region is noted for its floral diversity embedded in a highly dynamic system with significant spatio-temporal variations of physico-chemical variables preferably salinity Chaudhuri and Choudhury [6], Banerjee et al. [7], Mitra [4], Mitra and Zaman [5], Mitra & Zaman S [8], Pal et al. [9]. A glimpse of the spatial variation of physicochemical variables is highlighted in (Tables 1 & 2).Reflects the pattern of astaxanthin levels in six dominant mangrove species.
Table 1: Physico-chemical variables of aquatic environment of ten selected stations during postmonsoon, 2015. All readings are taken during low-tide condition.
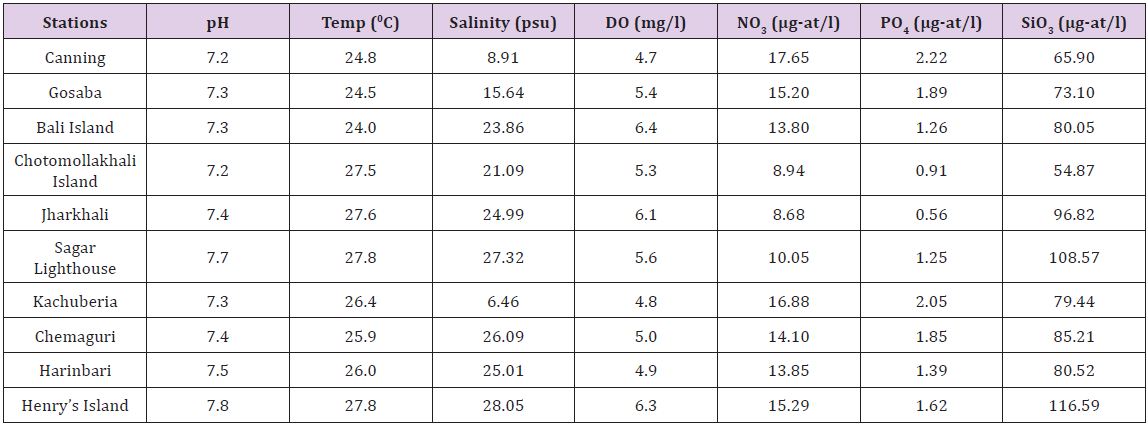
Table 2: Asthaxanthin content (ppm) in selective mangroves species collected from ten different selected stations during postmonsoon, 2015.
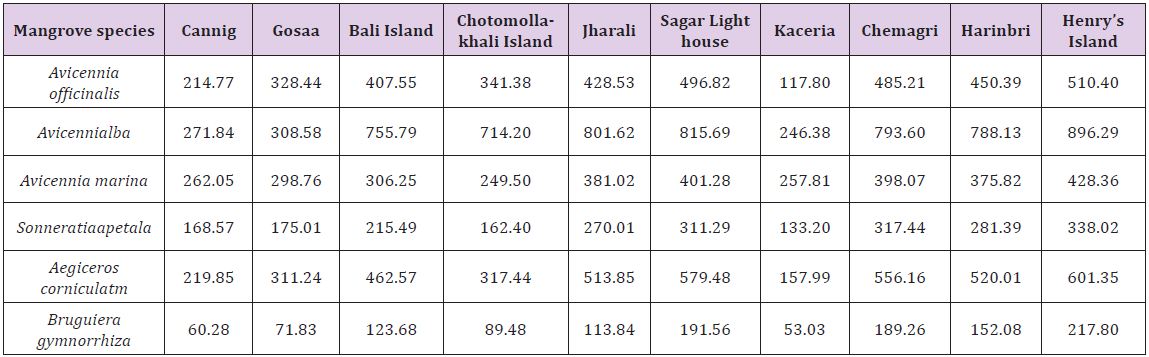
The relatively higher astaxanthin content in the leaves of mangrove plants confirms the synthesis of the pigment under stressful condition. The stressful condition arises due to continuous exposure of this vegetation under high saline condition. This is evident from the significant positive correlation values between astaxanthin levels in mangroves and the ambient aquatic salinity (p < 0.01). However, more studies are needed to establish the role of tidal influx and subsequent salinity fluctuation of the ambient aquatic phase on astaxanthin level in the mangrove floral parts (Table 3). The present data may serve as baseline information on the regulatory role of physico-chemical variables on astaxanthin level in the estuarine and coastal vegetation. The enhancement of astaxanthin production under stressed condition of organisms is a matter of interest and several researches are still being undertaken to pinpoint the reaction pathway of astaxanthin production by inducing stress of varied nature. The significantly high positive correlation of astaxanthin with salinity indicates that acceleration of astaxanthin production by mangroves may probably be a part of its adaptation to cope with the hypersaline condition of coastal and estuarine environment that is getting more acute with the climate change induced sea level rise in this mangrove dominated World Heritage Site.
Table 3: Inter-relationships between hydrological parameters and mangrove astaxanthin.