Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Wengui Shi1,2 and Jufang Wang1*
Received: April 11, 2017 Published: May 17, 2017
Corresponding author: Jufang Wang, Gansu Key laboratory of Space Radiobiology, Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou 730000, PR China
DOI: 10.26717/BJSTR.2017.01.000102
Microgravity in space can cause various problems in different biological systems. One of the most prominent and well recognized physiological challenges accompanying an extended spaceflight is the reduction in bone mass [1]. However, the underlying mechanisms of this phenomenon are still elusive. Primary cilium is a solitary and special organelle that emanates from the surface of most mammalian cells, which is anchored to the cell by mother centriole during the interphase and G0 of cell cycle [2]. For a long time, primary cilium was considered as a vestigial organelle. Until recently it was found that primary cilium provided a means of sequestering the centriole, so as to inhibit cell division. More significantly, a variety of receptors, ion channels and transporter proteins have been localized to the cilium, which has been proved as a key coordinator of signaling pathways to respond mechanical and chemical stimuli [3,4]. Primary cilium has been proved as a mechanosensor to regulate bone formation both in osteocytes and osteoblasts. It can act as a sensory organelle to receive extracellular signals and change its orientation to translate mechanical stimuli into biochemical and transcriptional changes, and as a result, bone formation is activated [5,6]. Inspired by established role as a mechanosensor, the role of primary cilium in microgravity induced bone loss must be studied. In here we presents the structure and function of primary cilium in bone metabolism process, and prospects the importance of primary cilia in microgravity stimulated osteoporosis.
Primary cilium consists of an axoneme of nine doublet microtubules that extends from the mother centriole of the centrosome, which is enclosed by the ciliary membrane, an extension of the cell membrane (Figure 1). The ciliary compartment is separated from the cytoplasmic matrix by transition fibers which connect the basal body (mother centriole) to the plasma membrane [2,7]. To build a primary cilium, the axonemal microtubules polymerize is delivered by intraflagellar transport (IFT). The IFT system is consisted by two associated cargo proteins, IFT-A, which is transported along axonemal microtubules by kinesin 2 motor proteins in the anterograde (base-to-tip) direction, and IFT-B, which is delivered by cytoplasmic dynein 2 in the retrograde (tipto- base) direction [8]. The overall protein composition of cilia is very complex, considering the function and structure of cilia. It has been estimated that cilia contain over 1,000 different proteins [9]. The tasks to confirm the functions of these proteins are still on the way (Figure 2).
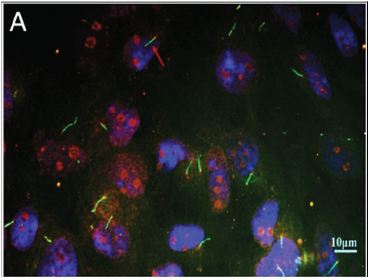
Figure 1: Immunofluorescence image of primary cilium.Primary cilium were stained by acetylated α-tubulin (green).The basal bodies were stained by γ-tubulin (red), and the nuclei were stained with DAPI (blue).
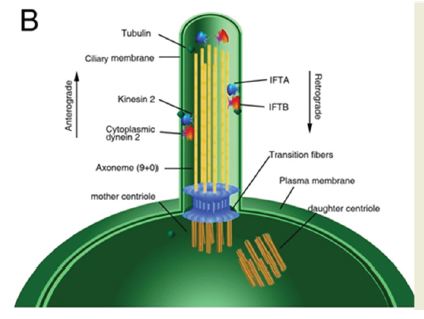
Figure 2:The schematic structure of primary cilium.
The signaling pathways coordinated by primary cilia are quite diverse and new pathways are identified by researchers every year. Except signaling like platelet-derived growth factor receptor (PDGFR), Wingless/Int-1 and Hedgehog (Hh)[10-12], more new primary cilium-located proteins were reported, including Transforming growth factor β (TGF-β ), protein kinase A, anoctamin and septin [13-15]. Xie et al. [16] reported that BMP-Smad1/5/8 signaling in osteoblasts was regulated by primary cilium through BMP receptor II (BMPRII) which was localized at the bases of cilia. Kwon et al. [17] found that dynamic flow transiently decreased cAMP production in a primary cilium dependent manner in osteocytes and adenylyl cyclase 6 (AC6) preferentially localized to the cilium [17]. Clement et al. [18] reported that TGF-β receptor localized to the ciliary tip and endocytic vesicles at the ciliary base in fibroblasts and that TGF-β stimulation increases receptor localization and activation of SMAD2/3 and ERK1/2 at the ciliary base [13]. Besides, many studies have shown that receptor tyrosine kinase (RTK) signalling pathways were involved in primary cilium, including PDGFRα signalling,IGF-1R signalling, EGFR signalling and FGFR signalling [18].
Over the past decade the primary cilium has emerged as a novel extracellular sensor in bone, being required to transduce changes in the extracellular mechanical environment into biochemical responses regulating bone adaptation. Malone et al. [6] report that bone cells possess primary cilia that project from the cell surface and deflect during fluid flow and that these primary cilia are required for osteogenic and bone resorptive responses to dynamic fluid flow. Hoey et al. [19] demonstrated that mechanically stimulated osteocytes secrete soluble factors which act in a paracrine manner to enhance osteogenic gene expression in MSCs. For in vivo studies, Temiyasathit et al. [20] demonstrated that deficient primary cilia in osteoblasts and osteocytes results in reduced loading induced bone formation and verifies earlier in vitro. Lee et al. [21] reported that knockout of AC6, which located in primary cilium, significantly reduced response to ulnar loading compared to WT rat. In addition, primary cilium also plays a key role in other bone promoters induced osteogenetic progress. For example, Yan et al. [22] found that primary cilia of osteoblasts play an indispensable role in mediating pulsed electromagnetic fields (PEMFs) osteogenic effect in vitro. Ma et al. [23] reported that primary cilia are important in mediating icaritin-stimulated osteogenic differentiation and may be a novel target for pharmacological therapies for bone loss.
Inspired by its established role as a sensory organelle, we hypothesize that primary cilium is a critical target of microgravity stimuli based on the following facts. Firstly, primary cilium is a mechanical stimulation sensor, which can change its orientation and length after the treatment of many force, like fluid shear stress and electromagnetic force [2]. If cells are exposed into microgravity condition for a long time, the primary cilium may change its length or morphology to adapt new environment. Secondly, on the one hand, it was found that primary cilium played an indispensable role in mediating normal bone formation induced by bone promoters. On the other hand, many studies have shown that microgravity can inhibit the differentiation, maturation and mineralization of osteoblasts and promoted bone loss [24].
Thus hypothesis that microgravity leads to bone loss because of the changes of primary cilium may be reasonable. Thirdly, reports had shown that the primary cilium can be as a gravitational force transducer. Moorman et al. [25] reported that primary cilium can detect the cyclical changes in the Earth’s gravitational field and transduce those changes into changes in the variability (stochastic nature) of gene expression. Finally, it has been found that microgravity affects the cytoskeleton in various cells, and it has been known that there are intrinsic connections between cytoskeleton changes and primary cilia. Dai et al. [26] reported that actin microfilaments participate in BMP-2 activity in activating Runx-2 and that their disruption might be an important contributor to microgravity-induced inhibition on BMP-2 activity in osteogenic induction [26]. Sharma et al. [27] reported that cytochalasin D increased the cilia length through changes in the actin network and in levels of soluble tubulin. Therefore, microgravity can affect actin cytoskeleton and it may change the primary cilium because of their intrinsic connections. In summary, future works need imminently to determine whether microgravity affects the length, orientation or morphology of primary cilia and whether these changes is responsible for the microgravity induced bone loss.


