Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Dayane Cristine Issaho1,2, Fábio Ramos de Souza Carvalho1, Geraldo de Barros Ribeiro3,Marcia Keiko Uyeno Tabuse1, Linda Christian Carrijo-Carvalho1, and Denise de Freitas1,
Received: January 20, 2018; Published: February 01, 2018
Corresponding author: Dayane C Issaho, MD, Coronel Dulcidio, 199, loandar, Zip 80.420-170, Curitiba, PR, Brazil
DOI: 10.26717/BJSTR.2018.02.000716
Purpose: This study aimed to analyze the inheritance pattern of infantile esotropia and to investigate the possible involvement of AHI1 gene.
Methods: The experimental group consisted of 14 neurologically normal patients with onset of large angle esotropia prior to 6 months of age. Three unaffected subjects from a family of twins with esotropia formed the familial control group. The main control group consisted of 11 organ donors with no history of strabismus. Familial pedigree charts allowed assessing transmission of strabismus phenotype up to four generations. Genomic DNA was obtained from peripheral blood and PCR amplification of AHI1 gene was performed.
Results: The mean onset of esotropia was 1.78±2.32 months and the mean deviation was 57±8.5 prims diopters. Amplification of AHI1 was observed in all patients in the control group, but not in those with infantile esotropia. Among the patients evaluated, 57% had a family history of strabismus. Genetic inheritance was observed in two families with monozygotic twins, one of them consisted of monozygotic twins with infantile esotropia and a third dizygotic twin without strabismus. AHI1 did not amplify in the 3 unaffected subjects from the triplets' family that formed the familial control group.
Conclusion: The family inheritance of congenital esotropia was verified in the individuals analyzed, with manifestation in the first, second or third generations. In addition, we reported the genetic inheritance in female twins and male triplets, with two univiteline twins presenting esotropia and another dizygotic who did not have strabismus. AHI1 gene may play a role in the pathology of infantile esotropia.
Abbreviations: DVD: Dissociated Vertical Deviation; SE: Spherical Equivalent; APCT: Alternate Prism-Cover Testing
Infantile or congenital esotropia is a constant non- accommodative convergent deviation with onset prior to six months of age in neurologically normal children. It is usually a large angle strabismus not related to refractive errors and often accompanied by other ocular motor abnormalities, such as inferior oblique overaction, dissociated vertical deviation (DVD) and latent nystagmus [1-3]. Treatment consists mainly in surgery or botulinum toxin injection in the medial recti muscles [3-5]. The prevalence of esotropia in the population is about 1%, while the prevalence of infantile esotropia reported at birth is 27 per 10.000 live births [1]. Its etiology is a source of controversy and remains unknown [1,4]. Esotropia is most prevalent in Europeans and their descendants, if compared to divergent strabismus. In Asia, exotropias are more prevalent [6]. In Brazil, a study performed in Sao Paulo identified esotropia as the most prevalent deviation, and it was more frequent in children younger than two years [7].
Infantile esotropia runs in families so there is a hereditary component, and primary monofixation syndrome occurs much more commonly in the parents and families of children with congenital esotropia than in the general population. 1 Although the genetic etiology of syndromic forms of strabismus have been described, the genes contributing to non-syndromic strabismus are currently unknown [8-10]. Significant genetic heterogeneity makes it difficult to establish the type of inheritance and related genes. Maumenee et al. [8] revealed a Mendelian co-dominant inheritance in infantile esotropia, with a high chance of affecting homozygotes with a relatively common allele. There is no evidence in the literature of the possible mutations, genes involved and intragenic location related to congenital convergent strabismus. It is known, however, that it is a complex inheritance [10]. Griffin et al. [11] found results consistent with a multifactor model (polygenic and environmental interaction).
AHI1 is a component of a protein complex in the cellular basal body, a ring-like structure that plays a role in the transition zone at the base of cilia. This complex acts as a barrier to restrict protein diffusion between plasma and ciliary membranes [12]. The International Radiation Hybrid Mapping Consortium mapped the AHI1 gene to chromosome 6. Mutations in this gene were identified in patients with Joubert syndrome type 3 [12,13]. Min et al. [14] have recently published results pointing AHI1 as an important target gene in strabismus. The present study reports the clinical findings and inheritance patterns in patients with congenital strabismus and among monozygotic and dizygotic twins. In addition, the putative role of AHI1 gene was evaluated.
This prospective study was approved by the Institutional Review Board at Federal University of Sao Paulo and complied with the principles mentioned in the Declaration of Helsinki. For the purpose of biological investigations, informed consent was obtained from all families of the individuals involved. The experimental group consisted of neurologically normal children with infantile esotropia operated for strabismus at Hospital Sao Paulo. Three unaffected individuals from a family with siblings presenting esotropia formed the familial control group. The main control group consisted of organ donors with no history of strabismus. A rigorous history of strabismus was taken for the organ donor control subjects. Patients undergoing surgery for infantile esotropia at Hospital Sao Paulo - Federal University of Sao Paulo - for a period of 6 months (from August 2013 through February 2014) were studied.
For inclusion, onset of esotropia must have been reported prior to 6 months of age and refractive error must be less than +3.0 spherical equivalents. Exclusion criteria were significant ocular co morbidities (e.g. cataract, optic nerve hypoplasia, laser treated ROP), and severe developmental delay or neurologic disorders (e.g. cerebral palsy, syndromic intellectual disability, seizures, etc.).
Patients with strabismus were analyzed for the following:
i. gender,
ii. age at reported onset of esotropia,
iii. age at surgery,
iv. visual acuity,
v. refractive error,
vi. pre-operative angle of Esotropia,
vii. presence of nystagmus, DVD, and/or oblique muscle overaction,
viii. presence of anomalous head turn;
ix. Pedigree chart.
Pre-operative vision was assessed with Teller visual acuity cards or Snellen. Refractions were performed after dilation with 1% cyclopentolate and 1% tropicamide, converted to spherical equivalent (SE) and averaged between the eyes for analysis. Amblyopia was defined as interocular difference of two lines or more in visual acuity or present with inability to maintain fixation through a blink or worse. Pre-operative deviation was measured with alternate prism-cover testing (APCT) at distance (6m), when possible, and at near in all patients (33cm). Krimsky measurements at near were used if APCT could not be obtained reliably. Oblique muscle dysfunction was graded on the standard 4-point scale.
Genomic DNA was obtained from peripheral blood samples collected with EDTA anticoagulant during strabismus surgery. DNA extraction from whole blood was performed with UltraClean Blood Spin DNA isolation kit (MoBio Laboratories, Inc., Carlsbad, CA, USA). The DNA was subsequently purified using the Power Clean Pro DNA Clean-Up kit (MoBio Laboratories, Inc., Carlsbad, CA, USA). The DNA samples obtained were analyzed and quantified by the ratio between absorbances 260/280 and 260/230 and standardized at a concentration of 50 ng/ml. PCR amplification was performed using a 20 μl volume containing 10 μl of DNA polymerase 2X Platinum SuperFi PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) with 4 μl of 5X SuperFi ™ GC Enhancer, and 1 μl of cDNA. Forward and reverse primers (bellow) were used at the final concentration of 0.2 μM. The reaction conditions were 95°C initial denaturation for 2 minutes, followed by 25 cycles of 20 seconds of denaturation, 20 seconds annealing and 45 seconds of extension at 95°C, 66°C and 68°C, respectively. The final extent occurred at 68°C for 5 minutes, followed by a final cooling step at 4°C.
Primer Forward: 5'- CAGGAAGGGAGCATAAAACTTACTTTCA-3'
Primer Reverse: 5'- TTCTCTGTGGTACAGTTGTGATAAAGC-3'
Five micro liters of each PCR product were analyzed by electrophoresis in 2% agarose gels and stained with the non- mutagenic fluorescent pigment EZ-Vision One DNA Dye (Amresco Inc., Solon, OH, USA). The gels were visualized under UV light, using the GeneRuler 100bp DNA Ladder (Thermo Fisher Scientific, Waltham, MA, USA) as standard. Data were analyzed using IBM SPSS Statistics v.20. Quantitative variables were described as mean, median, standard deviation, minimum and maximum. Categorical variables were described as percentages. P-values less than 0.05 were considered significant.
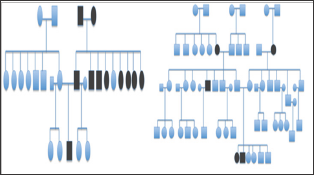
A total of 14 patients with infantile esotropia were included. Eight were female. From the female patients, two were monozygotic twins (Figure 1). Two of the male patients were monozygotic twins from triplets (Figure 2). Their parents and the third sibling without strabismus were also included in the genetic analysis. These 3 unaffected subjects formed the familial control group. The control group consisted on 11 organ donors with no history of strabismus. Altogether, 28 subjects were submitted to the genetic test. Esotropia onset was reported at a mean age of 1.8 ± 2.3 months (range 0-6 months). Mean age at surgery was 71 ± 64 months (range 22-231 months). Additional clinical findings are summarized in Table 1. Family inheritance of congenital esotropia could be verified in some of the individuals analyzed, in which strabismus manifested in the first, second or third generation. Pedigree charts of three families shown in Figures 3 and 4 demonstrate a presumptive autosomal dominant pattern. In the triplets' family, there was no history of strabismus in the previous first and second generations.
Table 1: Clinical findings in the group presenting infantile esotropia.
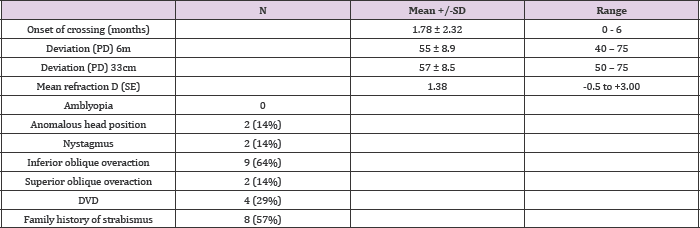
SD: standard deviation; PD: prism diopters; SE: spherical equivalent
Figure 1: Monozygotic twins with infantile esotropia.
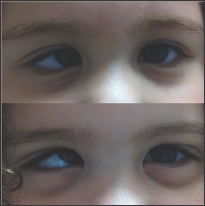
Figure 2: Monozygotic twins with infantile esotropia and third twin without strabismus.

Figure 3: Pedigree chart of monozygotic twins with infantile esotropia.
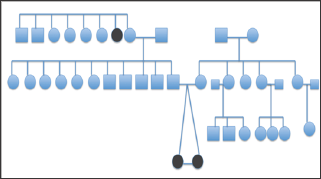
Figure 4: Pedigree chart of patients with family history of strabismus.
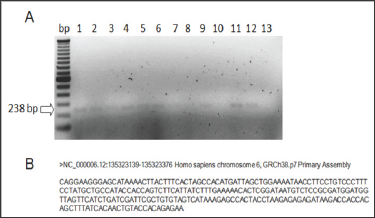
In the genetic analysis, AHI1 amplified only in the control group, which consisted on 11 organ donors with no history of strabismus (Figure 5). It did not amplify in any of the patients with infantile this type of strabismus. In the triplets' family the third triplet without deviation.
Figure 5: AHI1 amplification in the control group. A: Lanes 1-11: control group. Lanes 12: positive control of amplification. Lane 13: negative control of amplification (blank). B: Amplicon sequence retrieved from Primer Blast - NCBI.
Infantile esotropia was first well described by Costenbader in 1961 and is typically large angle strabismus (>30PD), unrelated to refractive error, often associated with other ocular motor abnormalities, such as dissociated vertical deviation (DVD), inferior oblique overaction and nystagmus [2,3,15]. Several studies revealed the presence of amblyopia in 40-50% of the children with infantile esotropia [3,16,17]. In our series, none of the 14 patients presented amblyopia at the time of the exam. The incidence of DVD (29%) was similar, although inferior oblique overaction (64%) was somewhat higher in our patients than in other published series, but this likely relates to the elder age of our group [3,16,18,19]. Over the past decades, whole genome sequencing efforts are progressing to determine the elusive strabismus genes. This study intended to contribute with new genetic findings on non-syndromic esotropia. Infantile esotropia runs in families so there is a hereditary component. In our series, family history of strabismus was observed in 57% of the patients with infantile esotropia, although the inheritance pattern could not be defined.
A presumptive autosomal dominant pattern was observed in three families. Squint was reported in the previous first, second or third generations. The overall incidence and occurrence of specific types of strabismus show considerable differences between racial groups, advocating that the etiology of strabismus has genetic factors. In 2003, Parikh et al, evaluating all individuals in a family with non-syndromic strabismus, demonstrated the susceptibility of mutation in the 7p22.1 gene in a recessive inheritance model [20]. The same authors did not observe a significant association with the gene 7p22.1 in six other families, thus establishing a possible genetic heterogeneity. These observations suggest the hypothesis about the various point mutations that may be associated with congenital esotropia. However, the aspects are poorly explored in the experimental scientific study on the main molecular and biochemical factors involved in congenital esotropia. In this study, the female monozygotic twins presented the same clinical findings (age at onset, refraction and angle of deviation). Parents were unaffected with strabismus; however a father’s aunt had a history of esotropia.
The male monozygotic twins with strabismus also presented the same clinical exam. They had a history of sharing the same placenta in the prenatal period. The third sibling, who did not manifest strabismus, had a different placenta. Parents were also unaffected and there was no family history of strabismus in the previous first and second generations. This is the first report in the literature that shows simultaneous observation in monozygotic twins with unaffected parents and dizygotic sibling manifesting similar genetic pattern, reinforcing the genetic basis of strabismus. The constitution of the AHI1 gene contains at least 33 exons and spans 213.7kb [12]. Mutations in this gene were identified in patients with specific types of Joubert syndrome-related disorders [13]. Pathogenic variants in AHI1 were identified in 7.3% of 137 individuals with Joubert syndrome [21]. Additionally, about 80% of them had retinal dystrophy [22]. Herein, genetic analysis also points to AHI1 as a potential target associated with congenital esotropia, possibly through an additive effect. AHI1 amplified in all the patients of the control group and it did not amplify in any of the individuals with infantile esotropia and the familial control group.
Min et al. have recently published results that corroborates with ours, pointing AHI1 as an important target gene in strabismus [14]. They investigated a Chinese strabismus pedigree with unaffected parents. By whole-exome sequencing, 2 mutations c.A3257G (p.E1086G) in AHI1 and c.A914G in NEB were identified as the most likely causative mutations in the disorder. As genome sequencing efforts are advancing for the discovery of the unknown strabismus genes, results obtained in this study suggest a possible gene target involved in strabismus. In addition, the present findings correlate with the clinical and family pattern of infantile esotropia associated to autosomal inheritance, which may have a multifactorial character.
The family inheritance of congenital esotropia could be verified in the individuals analyzed, with manifestation in the first, second and third generations. In addition, the genetic inheritance was observed in female twins and male triplets, with two univitelines presenting esotropia and another dizygotic who did not have strabismus. AHI1 gene may play a role in the pathology of infantile esotropia. Additional research is needed to elucidate the genetic patterns of this disorder.
Supported by Sao Paulo Research Foundation (FAPESP) Grant 2011/51626-1 and Coordination for the Improvement of Higher Education Personnel (CAPES), Ministry of Education of Brazil. Fellowship support received by CAPES (to DCI), National Postdoctoral Program (PNPD)/CAPES (to LCC-C), Young Researchers at Emerging Centers Program of FAPESP, Grant 2012/15603-0 (to FRdSC), and a research productivity fellowship from the Natio al Council for Scientific and Technological Development (to DdF).


