ABSTRACT
The SARS CoV-2 is responsible for the severe acute respiratory syndrome (COVID-19) which has claimed numerous victims worldwide. The main symptoms reported in the scientific literature are represented by fever, cough, gastrointestinal disturbances, dysgeusia, and anosmia but currently the symptomatology is not clear, yet. The symptoms vary from one individual to another one, depending mostly on the age of the patients and their co-morbidities. Wearing surgical or FFP2/KN-95 face masks block the interpersonal transmissibility of the droplets containing the virus which are produced during speaking, breathing or coughing. We pondered the regeneration of this personal protective equipment in order to reduce the costs and the biological waste released into the environment. In our paper, we have described four strategies for the decontamination of surgical and FFP2/KN95 face masks. The steam iron and the washing machine are easily reproducible at home, while the thermostatically controlled bath and the autoclave require more advanced structures such as hospitals or scientific institutes. The regeneration methods ensure that both surgical and FFP2/ KN95 face masks maintain the same characteristics of the unused ones. After the regeneration process, we demonstrated the conservation of the structural proprieties of the polypropylene fibers of both masks by light microscope. The microbiological analyses of both regenerated masks showed the absence of the main pathogens that can infect the nasopharyngeal tract. Moreover, we showed that both regenerated surgical and FFP2/KN95 face masks maintained their waterproof properties. Finally, we demonstrated that the regenerated FFP2/KN95 face masks maintained their adequate fit by the qualitative fit testing.
Introduction
The health emergency caused by SARS-CoV-2 has required intervention measures for respiratory protection in healthcare workers and generally in the community, to prevent the spread of the viral infection. SARS-CoV-2 is a positive-sense, single-stranded RNA virus that is highly contagious [1]. The virus uses specific proteins (S-Proteins) to attach certain kinds of receptors in the epithelial cells of the respiratory tract [1]. The S protein mediates the viral entry into the host cells by binding to the host Angiotensinconverting enzyme 2 (ACE2) receptor which is highly concentrated in the airway epithelial cells. Droplets and aerosol particles produced during breathing may contain the viral load in variable quantities [2]. The SARS- CoV-2 could stay viable for 3h in aerosols highlighting the crucial role of the use of the face masks to avoid the infection [3]. Some studies demonstrated that the SARS CoV-2 could survive for 72h on plastic, from about 4 to 48h on metallic surfaces, and for 24h on cardboards [3]. Moreover, the SARS- CoV-2 is not similar to the influenza or other common Coronavirus as the spread of the viral infection is not blocked by warm weather, and according to the latest research, the virus outbreaks along restricted latitudes, temperature, and humidity [4].
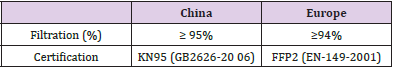
Some epidemiological studies showed that for human beings the most common risk factors includes age, male sex, obesity, smoking, and co-morbid chronic conditions such as hypertension, type 2 diabetes mellitus, and others [5-7]. As usual during initial phases of pandemics it turns to be crucial to develop a contingency plan to compensate shortage of consumables due to the ramp up of the contagious [8,9]. As a matter of fact, some strategies to decontaminate and reuse the masks several times are needed. These methods should be performed in total safety with the aim to reduce the costs associated with the purchase of the protective devices. Moreover, the regeneration of the masks reduces the polluting impact on the environment [10]. The decontamination procedure should be done adequately in order to preserve the filtration proprieties [11]. A surgical mask is designed to protect the wearer from splash or spray while the filtration capacity is variable depending on the materials and the number of layers. The surgical mask does not fit around the face at the superior level compared to other type of masks such as the FFP2 face masks. Indeed, differently from the surgical masks, the FFP2-face masks have filter capacity ≥ 94% of airborne particles (≤ 5 μm in diameter) whereas the KN95- face masks have filter capacity ≥ 95% of airborne particles (Table 1). Both masks fit well around the face of the wearers [12]. The FFP2/KN95 face masks consist of a number of variable layers of fabric (multilayer masks) and they have generally four or six layers. The filtration grades for KN95-face masks (made in China) are quite similar to the FFP2 respirators (European Union).
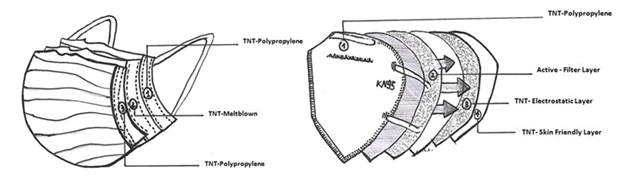
A standard surgical face mask has 0.3-10 μm pore size while Coronavirus has a diameter from 0.06 to 0.14 μm and it has got a spherical shape [13]. The surgical masks should not be used by the healthcare workers who are in contact with COVID-19-positive patients. In this case, the healthcare workers ought to wear the FFP2/ KN95 face masks. Most of the filtering materials used for the FFP2 face masks are synthetic, such as non-woven fabrics (TNT) based on materials as polypropylene (PP), polyethylene terephthalate (PET), and polyamide (PA). The outer layer is usually made of TNT spunbond (S) which is treated with hydrophobic treatment while the intermediate layer is often made of TNT meltblown (M) which performs the main filtering function of the mask. The inner layer, which is in contact with the face and protects the skin from the filtering layer, is made of spunbond [14]. The meltblown layer is composed of polypropylene microfibers with variable diameters (from 1 to 10 μm) which create a three-dimensional network Figure 1. Our aim is to suggest strategies for the decontamination of disposable respiratory devices for the community or the hospital settings. In particular, we focused on methods for the regeneration of surgical and FFP2/KN95 face masks (without respiratory filters) used in companies as well as at home. Furthermore, a large chapter should be reserved to numerous counterfeit certification masks present on the world market during pandemic period, considering that these masks lead to the increasing of new cases [15].
Figure 1:
a. Comparison between the different layers and materials used to make the surgical masks and
b. The FFP2/KN95 face masks.
Materials and Methods
Samples
We focused on the regeneration of two types of disposable masks: surgical masks (Jiangsu Province Jianerkang Medical Dressing Co., LTD China) and FFP2/KN95 face masks (KN95 Yiwubiweikang labor protection products Co., Ltd-China and FFP2 BLS 502 PPE-R/02.075 version 1 COVID-19, Italy). We tested a total of 24 masks: 12 surgical masks and 12 FFP2/KN95 face masks.
The Regeneration Methods for both Surgical and KN95/FFP2 Face Masks
Thermostatically Controlled Water Bath
We tested 2 surgical masks and 2 KN95/FFP2 face masks which were worn by the operator for about 8 hours. The masks were immersed in hot water thermostatically controlled bath/5 Liters (VWR International S.r.l, Italy) at 80°C (constant temperature). The temperature was monitored by the display on the thermostat bath and a mercury thermometer inserted in the tank of the water bath. The masks were soaked in the water bath at different times (15 minutes, 30 minutes, and 120 minutes), and immediately after, they were shaken to remove of water and air-dried.
Washing Machine
We tested 4 surgical and 4 KN95/FFP2 face masks, worn by the operator for about 8 hours and we washed them using a common laundry detergent in a quick washing cycle machine (LG Electronics S.p.a, Italy) at 60°C for 30 minutes.
Steam Iron
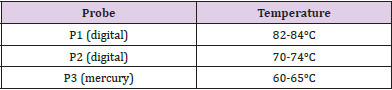
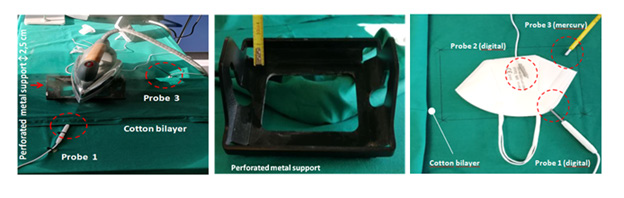
We analyzed 4 surgical and 4 FFP2/KN95 face masks worn by the operator for about 8 hours which were regenerated using a domestic steam iron machine; in particular, we used Vaporella Forever 615 P:4 Bar 80 G/min (Polti SPA, Italy). The masks were not placed in direct contact with the surface of the steam iron but they were placed under a double layer of cotton obtained by folding a cloth (Figure 2). The iron was placed on a 2.5 cm high perforated metal support (0705/04 centrifuge plate adapter (Eppendorf-S.r.l, Italy). The support was arranged above the double layer cotton in order to allow the steam to radiate correctly into the area containing the mask. Two digital and one mercury thermometers (probes) were used to detect the temperature reached in the different points of the masks. The first probe (P1) was positioned inside the masks (area of nose and mouth) while the second probe (P2) was positioned between the outer layer of the masks and the cotton double layer. Finally, the third probe (P3) was placed in the area surrounding the steam iron (Figure 2). The steam flux was sprayed over the area where the masks were placed for 35 minutes, and the temperatures detected are shown in the Table 2.
Autoclave
We tested 4 masks and 4 FFP2/KN95 face masks worn by the operator for about 8 hours and we regenerated all of them using an autoclave in our laboratory. The masks were wrapped in a single layer of aluminum foil (used for food) and placed inside the autoclave (Vapormatic 770-Asal S.r.l, Italy) for a full cycle at 121°C 1 ATM for 35 minutes.
Tests to Assess the Effectiveness of Regeneration Procedures
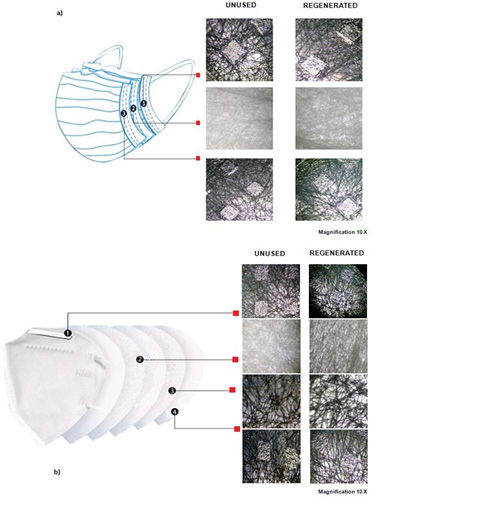
Evaluation of the Fibers by Light Microscope: We examined 24 masks (12 surgical and 12 FFP2/KN95 face masks). For this purpose, we carried out an optical microscope survey for each regeneration method (thermostatically controlled water bath, washing machine, steam iron, and autoclave) to verify any possible potential damage to the fibers caused by these procedures. The surgical masks are usually made up of three layers (internal, intermediate, and external) and we analyzed all the layers by the excision of a tissue fragment of about 0.5 cm2 using a scalpel. Each fragment was placed on a microscope slide, covered with a slide, and observed at 10x magnification (Figure 3a). The same procedure was repeated for the four layers of the FFP2/KN95 face masks (Figure 3b).
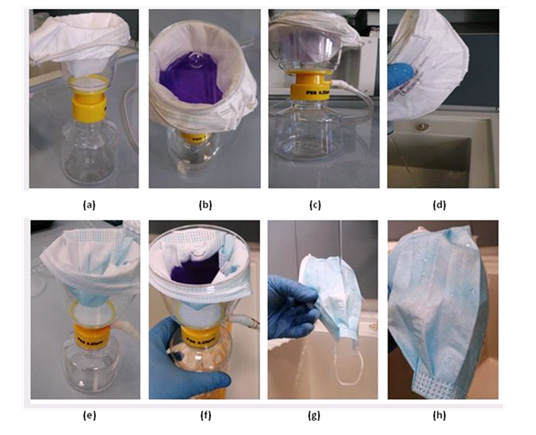
Evaluation of the Waterproof Property: We tested 24 masks (12 surgical and 12 FFP2/KN95 face masks). The water repellent properties of the masks represent a crucial point that is essential to prevent and blocking microorganisms and viruses that can be contained in the droplets released during conversation and breathing. Primo vacuum filter system (250 mL-0.22 μm PES Hydrophilic 1/pkg, Euroclone, Italy) was used to test the maintenance of the waterproof properties. A vacuum filter system was a common method used previously for the sterile filtration of laboratory reagents, buffers, solutions and media. Each type of mask was put inside the Vacuum Filtration Tops with the outer surface facing upwards (Figures 4a-4e). We obtained 500 milliliters of coloured solution by mixing 250 mL of crystal violet and 250 mL of distillate water. We poured the colored solution inside the outer surface of the masks (Figures 4b-4f). Finally, the vacuum aspirator was activated and we observed the inner surface of the masks to determine their waterproofness (Figure 4c). Finally, both surgical and FFP2/KN95 face masks were subjected to a flow of running water to determine their wettability (Figures 4d, 4g & 4h).
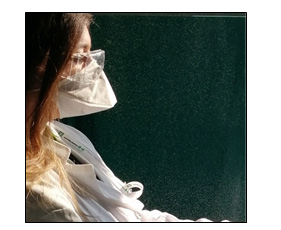
Qualitative Fit Testing: This test was conducted to evaluate the ability of 4 surgical and 4 FFP2/KN95 face masks to filter test agents aerosolized in the air (Figure 5). The substances used as testing agents had a pungent (wine vinegar), bitter (coffee), and sweet (vanillin) taste-odor. We aimed to verify the absence of a specific flavor in the mouth or nose. The qualitative fit test had the purpose of evaluating the ability of the masks to maintain an adequate fit before and after regeneration [16]. In addition, this experiment was done following the OHSA (Occupational Safety and Health Administration) guidelines which indicate a series of physical exercises to emulate the work activity while wearing the mask, and to reproduce the stresses associated with the movement of the workers. While standing, the subject was asked to perform a series of 1-minute exercises: normal breathing, deep breathing, turning head side to side, moving head up and down, reading from a prepared text, jogging in place, and normal breathing [14,17,18].
Figure 3:
a) Comparison between the layers of an unused surgical mask and a steam regenerated mask.
b) Comparison between the layers of an unused FFP2/KN95 face mask and a steam regenerated mask.
Figure 4: Waterproof test procedure. The first line represents the procedure used for the FFP2/KN95 face masks while the second line represents the procedure used for the surgical masks.
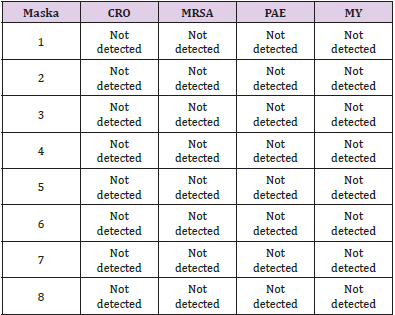
Evaluation of the Efficacy to Inactivate Microbiological Agents: The microbiological tests were been carried out using eSwabs 480C (Copan Diagnostic Inc., California, USA). We tested four regenerate surgical and four regenerated FFP2/KN95 face masks. The procedure was performed under a sterile hood in order to avoid any potential cross contamination of whatever environmental nature. We previously moistened the inner surface of each mask with a sterile physiological solution (Sodium Chloride 0.9% B. Braun Melsungen AG, Germany). Then, the flocked swabs were rubbed along the entire internal surface of the surgical and FFP2/KN95 face masks. Immediately after sampling, we placed the flocked swabs inside the vials containing liquid amies preservation medium. We performed the microbiological investigations searching for the followings strains: Gram negative bacteriae with resistance to carbapenems (CRO), Staphylococcus Aureus resistant to methicillin (MRSA), Pseudomonas Aeruginosa (PAE), and Medical Yeasts (MY). It should be stated, therefore, that to pass the swab (pharyngeal type) inside the regenerated masks and conducing tests could not totally guarantee that the regeneration is absolutely good, due to the fact that the above-mentioned colonies could be not present on the inner surface of those used masks prior to the regeneration.
Results
Evaluation of the Fibers by Light Microscope
The regenerated masks (surgical and FFP2/KN95 face masks) analyzed under the light microscope showed the original weave as well as the morphology by observing all the layers (Figures 3a & 3b), therefore in the future we expect do extend experimentations with quantitative analysis (e.g., pore measures, filament diameters).
Evaluation of the Waterproof Property
Both surgical and FFP2/KN95 face masks are suitable for directly blocking the droplets during breathing. We demonstrated that the regenerated masks contained useful properties. Indeed, the water repellency remained unchanged in the regenerated masks with respect to the unused masks, even if we should outline that «water repellency», alias hydrophobicity, is measured with the contact angles and it is not synonymous with being fully «waterproof» when the dye passes through the vacuum filter (Figures 4d, 4g & 4h).
Qualitative Fit Testing
We demonstrated that the surgical masks due to their conformation were not able to isolate the operator from the environment odours because they do not perfectly adhere to the face before and after the regeneration process. On the other hand, we observed that the unused FFP2/KN95 face masks as well as the regenerated ones were able to partially block the smells. Thus, our qualitative fit test showed that the FFP2/KN95 face masks remained comparable to the unused masks.
Evaluation of the Efficacy to Inactivate Microbiological Agents
The microbiological evaluation tests came back negative to the CRO, MRSA, PAE & MY (Table 3).
Table 3: Microbiological tests of the regenerated surgical masks (1,2,3,4) and FFP2/KN95 face masks (5,6,7,8).
Discussion
Despite the discovery of vaccines, our research focuses on two main issues: the wastefulness of economic resources and the ecological environment dangers connected to the waste masks. For these reasons, we decided to report four easy and cost-effective approaches to test both surgical and FFP2/KN-95 face masks in order to reuse them several times. As a matter of fact, in the global market there are several types of masks, and the choice of the mask depends on the use made by the operator. We focused on both surgical and FFP2/KN95 face masks because these two kinds of masks were easily to find on the market during the pandemic. Our experimental tests showed that our methods used to regenerate surgical and FFP2/KN95 face masks, did not damage the fibers of the masks suggesting that the regeneration methods are suitable. The decontamination procedures used to regenerate surgical and FFP2/KN95 face masks are scientifically validated by the current literature [19-21]. Abraham et al. reported the proper sterilization of the physical objects that have been contaminated by SARS-CoV-2 [22]. In agree with the World Health Organization (WHO) guidelines, the authors comply with a 4log reduction of the viral load (SARSCoV- 2) with a treatment at 56°C for 15-minutes exposures (www. who.int/csr/sars/survival_2003_05_04/en/).
Starting from this premise, we decided to exploit four regeneration methods based on heating treatments as follows: thermostatically controlled water bath, washing machine, steam iron, and autoclave. We chose to increase the exposure time of all the heating treatment of the masks as well as the temperature, in order to ensure the elimination of the SARS CoV-2 from the treated masks. Wang et al. have already done a procedure to decontaminate the masks using a tank of hot water bath. Instead, our thermostatically controlled water bath was based on three hot water soaking cycles (15 minutes, 30 minutes, and 120 minutes). Compared to the investigations made by Rowan who showed a method to regenerate face cloths by using a washing machine, we applied the same method to four surgical and four FFP2/KN95 face masks using a softening detergent that improved the adhesion to the face. We would like to point out that using a domestic washing machine cycle at 60°C, the power of the decontamination is not only represented by the high temperature but also by the addition of a detergent which can inactivates viruses (https://www.cdc.gov/ coronavirus/2019-ncov/community/disinfecting-building-facility. html, Abraham JP). As far as we know, our steam iron method used for the regeneration of the masks is innovative and it is easily accessible by a large part of the community. Indeed, the metal support used in our test in order to avoid a direct contact between the iron and the surface of the mask, can be easily emulated using a common kitchen tool such as a rectangular perforated steel hot pad.
As well, our autoclave regeneration method which was applied to both surgical and FFP2/KN95 face masks was supported by Seresirikachorn et al. but with different results. The authors used the same procedure to decontaminate the N95 FFRs masks. After a full cycle in an autoclave at 121°C, the outer layers of N95 FFRs masks were deformed. On the contrary, we tested four surgical and four FFP2/KN95 facial masks for a full cycle at 121°C/ at 1 ATM for 35 minutes, and we did not observe any morphological change in the fibers of all the masks using the light microscope. Moreover, we decided to check the absence of microbiological agents in the regenerated masks in order to assess the safety of our methods of decontamination.
We analysed the more common microorganisms such as Gram negative/Gram positive bacteriae and Medical Yeasts which can infect the nasopharynx tract. For each regeneration mask, we carried out a microbiological test which demonstrated that all the masks were free of microbiological loads. Notably, we performed the four regeneration processes on each mask twice. Thus, each regenerated mask was worn for about 8 hours and then they were regenerated for the second time. These latest masks did not show any physical deformations, nether alteration in the elastic bands nor the metal strips. As far as we know, there is no data regarding the re-use of a mask after processes of regeneration by steam iron procedure as shown above. The critical point of all the four regenerative methods concerns the removal of the make-up residues. The washing machine and the thermostatically controlled water bath were not able to remove the stains of cosmetics from the masks. Not even, the high temperatures reached with the regeneration methods such as the autoclave (121°C) or the steam iron (82°C - 84°C), were able to eliminate the spots. On the other hand, we treated both stained surgical and FFP2/KN95 face masks with acetone and a degreaser to remove the makeup residues but with poor results.
In conclusion, we highlighted that all the regeneration methods with the exception of the autoclave, can be emulated at home, hospital, or research institutes. This makes it possible to greatly reduce the costs associated with the purchase of new masks and the amount of the biological waste released into the environment. In addition, our regenerative methods offer the community to sanitize surgical and FFP2/KN95 face masks at least twice, thus extending the half-life of the masks. Finally, we have introduced the steam iron as a new regenerated procedure among the common regeneration methods.
We are currently suggesting introducing our regeneration methods such as the autoclave and the washing machine as standard decontamination procedures of the surgical and FFP2/ KN95 face masks for healthcare workers in our public hospital in Milan (Italy).
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the research, authorship, and/or publication of this article.
Acknowledgment
We would like to thank Fondazione Malattie del Sangue (FMS) Onlus for its support.
References
- Malik YA (2020) Properties of Coronavirus and SARS-CoV-2. Malays J Pathol 42(1): 3-11.
- Seresirikachorn K, Phoophiboon V, Chobarporn T, Tiankanon K, Aeumjaturapat S, et al. (2021) Decontamination and reuse of surgical masks and N95 filtering facepiece respirators during the COVID-19 pandemic: A systematic review. Infect Control Hosp Epidemiol 42(1): 25-30.
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, et al. (2020) Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med 382: 1564-1567.
- Sajadi MM, Habibzadeh P, Vintzileos A, Shokouhi S, Miralles-Wilhelm F, et al. (2020) Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19). SSRN [Preprint] 9: 3550308. Update in: JAMA Netw Open 3(6): e2011834.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, et al. (2020) Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 180: 934-943. Erratum in: JAMA Intern Med 180(7): 1031.
- Garibaldi BT, Fiksel J, Muschelli J, Robinson ML, Rouhizadeh M, et al. (2021) Patient Trajectories Among Persons Hospitalized for COVID-19: A Cohort Study. Ann Intern Med. 2021;174: 33-41. Erratum in: Ann Intern Med 174(1):144.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054-1062. Erratum in: Lancet 395(10229): 1038.
- Bossomaier T, Bruzzone AG, Massei M, Newth D, Rosen J, et al. (2009) “Pandemic dynamic objects & Reactive Agents. Proceedings of MAS2009, Tenerife, September.
- Einav S, Hick JL, Hanfling D, Erstad BL, Toner ES, et al. (2014) Surge capacity logistics: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. CHEST 146(4): 17S-43S.
- Patrício Silva AL, Prata JC, Walker TR, Duarte AC, Ouyang W, et al. (2021) Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem Eng J 405: 126683.
- MacIntyre CR, Chughtai AA (2015) Facemasks for the prevention of infection in healthcare and community settings. BMJ 350: h694.
- Bhattacharjee S, Bahl P, Chughtai AA, MacIntyre CR (2020) Last-resort strategies during mask shortages: optimal design features of cloth masks and decontamination of disposable masks during the COVID-19 pandemic. BMJ Open Respir Res 7(1): e000698.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2019) China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 382: 727-733.
- Santarsiero A, Ciambelli P, Donsì G, Quadrini F, Briancesco R, et al. (2020) Face masks. Technical, technological and functional characteristics and hygienic-sanitary aspects related to the use of filtering mask in the community. Ann Ig 32(5): 472-520.
- Lam SC, Suen LKP, Cheung TCC (2020) Global risk to the community and clinical setting: Flocking of fake masks and protective gears during the COVID-19 pandemic. Am J Infect Control 48(8): 964-965.
- Regli A, Sommerfield A, von Ungern-Sternberg BS (2021) The role of fit testing N95/FFP2/FFP3 masks: a narrative review. Anaesthesia 76(1): 91-100.
- Oberg T, Brosseau LM (2008) Surgical mask filter and fit performance. Am J Infect Control 36(4): 276-82.
- (1998) Respiratory protection--OSHA. Final rule; request for comment on paperwork requirements. Fed Regist 63(5): 1152-300.
- Wang D, Sun BC, Wang JX, Zhou YY, Chen ZW, et al. (2020) Can Masks Be Reused After Hot Water Decontamination During the COVID-19 Pandemic? Engineering (Beijing) 6: 1115-1121.
- Rowan NJ, Laffey JG (2021) Unlocking the surge in demand for personal and protective equipment (PPE) and improvised face coverings arising from coronavirus disease (COVID-19) pandemic - Implications for efficacy, re-use and sustainable waste management. Sci Total Environ 752: 142259.
- Seresirikachorn K, Phoophiboon V, Chobarporn T, Tiankanon K, Aeumjaturapat S, et al. (2021) Decontamination and reuse of surgical masks and N95 filtering facepiece respirators during the COVID-19 pandemic: A systematic review. Infect Control Hosp Epidemiol 42(1): 25-30.
- Abraham JP, Plourde BD, Cheng L (2020) Using heat to kill SARS-CoV-2. Rev Med Virol 30(5): e2115.

 Research Article
Research Article