ABSTRACT
Chlamydia trachomatis (CT) infection in the genitourinary tract is the most prevalent bacterial sexually transmitted disease (STD) worldwide. Genital chlamydial infection has a huge impact on sexual and reproductive health, and it is very common in developed and developing countries. The aim of this study is to determine the prevalence of Chlamydia trachomatis antigen and identify associated risk factors among HIV-positive males on Highly Active Antiretroviral Therapy (HAART) in Lagos State, Nigeria. A total of 318 male subjects (159 HIV positive and 159 HIV negative) between the aged of 18-50 years and above were recruited for the study. A structured questionnaire was administered to the study participants to collection their sociodemographic and clinical information. Urine sample was collected from each participant and were analyzed for the presence of CT antigen using One-Step Chlamydia Rapid Diagnostic Test kit (GIMA, Italy) and the results were interpreted according to the manufacturer interpretation guide. Out of the 159 test samples of the HIV positive men analyzed for uro prevalence of CT antigen, 5 (1.6%) were positive for CT antigen, while 154 (48.4%) were negative. Meanwhile, out of the 159 control samples of the HIV negative men analyzed, only 1(0.3%) person was found to be positive for CT antigen. The remaining 158 subjects (49.7%) were negative. Risk factors that are very important for the occurrence of CT antigen amidst the study participants include: marital status (P=0.013) and number of sexual partner (P=0.014). The outcome of this study shows that CT antigen (1.6%) exists among HIV positive men attending the HIV clinic at National Institute of Medical Research (NIMR), Yaba, Lagos State. Hence, the clarion calls for the facility to include C. trachomatis antigen testing in their routine screening for HIV patients.
Keywords: Chlamydia trachomatis Antigen, Risk Factors, HIV Positive Men, Lagos State, Nigeria
Introduction
Chlamydia trachomatis, the aetiology of chlamydia, is a coccoid bacillus that is closely linked to Gram-negative bacteria (Cheesbrough [1]). The organism is a member of the Chlamydia genus, which also contains organisms formerly referred to as PLT (Psittacosis Lymphogranuloma venerum Trachoma group) or TRIC (Trachoma Inclusion Conjunctivitis group) species (Collee, et al, [2]). There are fifteen (15) serotypes of Chlamydia trachomatis. These include L1-L3 which causes lymphogranuloma venerum (associated with genital ulcer disease in tropical countries), D-K which causes genital tract infection and trachoma (chronic conjunctivitis common in Africa and Asia). Chlamydia trachomatis serovars D-K are responsible for the treatable STD Chlamydia (CDC [3]). One of the most common sexually transmitted diseases in the globe is the illness that results from an infection of the lower genital tract (Gerbase, et al. [4]; Beagley and Timms, 2000). The World Health Organization (WHO [5]), estimates that 101 million chlamydial infections occur each year globally. According to the Centers for Disease Control and Prevention, 2.8 million Americans contract the disease annually (CDC [3]). More than 90% of the population in some thirdworld nations is infected with Chlamydia trachomatis (Gomes, et al. [6]). Chlamydial infection is often without symptoms; in fact, 50% of men and 80% of women do not exhibit any symptoms, which is why it is known as the “silent disease” (Gaydos et al., 2018; CDC [3]; Wodarz and Hamer [7]). If symptoms are present, they might merely last a few days, go unnoticed, or not be given much weight (Kidshealth, 2006). Itching in the urethra, frequent and painful urination, sores on the penis, scrotum, or anus, penile itching, and discharge that may be watery, white, or hazy are among the clinical signs in males when they are present. Infection can virtually spread to any part of the body (lungs, heart, eyes, muscles, prostate gland, etc.). Impotence is a critical complication of chlamydial infection in men (CDC [3]; Al-Mutairi, et al. [8]).
Chlamydial infections that are symptomatic lead to a two- to five-fold increase in HIV transmission and acquisition. Chlamydial blisters and sores can worsen HIV infection, lower CD4 counts, and increase viral loads in patients who already have the virus. On the other hand, having HIV makes one more likely to get a neurochlamydial infection. It might affect chlamydial’s clinical characteristics and treatment outcomes. HIV infection might be linked to treatment failure for chlamydial, particularly if neurochlamydial was diagnosed later than expected. Additionally, Chlamydia can mimic several clinical manifestations and harm HIV-positive patients’ cardiovascular and neurological systems severely. Epidemiological research has also demonstrated that untreated genital Chlamydia infection increases the likelihood of heterosexual Human Immunodeficiency Virus (HIV) transmission. Consequently, CTI screening in high-risk populations can help with the development of HIV risk reduction strategies (Joyee, et al. [9]). It is a known fact that Chlamydia causes localized inflammations and immunological reactions that are marked by the increase and infiltration of immune cells with CD4 surface proteins necessary for HIV binding prior to entrance, which also makes HIV more likely to enter the body (Altes et al., 2012; Joyee, et al. [10]; Wodarz and Hamer [7]) and therefore an important risk factor for the development of genital tracts infection among the HIV infected individuals. This is not unconnected to the weak immune status of such individuals. However, since infection with C. trachomatis is effortlessly curable, early detection and treatment of infected sufferers is important to halt the cycle of infection among the populace (Haggerty, et al. [11]).
Even though it is treatable, Chlamydia trachomatis genital infection is one of the most common bacterial sexually transmitted diseases (WHO [12]; Patel, et al. [13]). The WHO estimates that 92 million new cases of C. trachomatis infection occur annually worldwide, with roughly two-thirds of these infections occurring in underdeveloped countries with limited access to diagnostic and treatment options (Patel, et al. [13]). The majority of Chlamydia trachomatis infection (CTI) epidemiological data comes from industrialized countries. Unfortunately, trustworthy data from resource- poor developing nations with high burden of the disease is not readily available. However, despite this challenge in data gathering, it’s still crucial to scientifically document the frequency and prevalence of CTI from the poor world using laboratory tests. To the best of our knowledge, no work has been done to assess the prevalence of Chlamydia trachomatis antigen among HIV positive men on HAART attending the HIV clinic at the Nigerian Institute of Medical Research (NIMR), Lagos state, Nigeria. Besides there is need to identify risk factors that predispose male individuals in this setting to Chlamydia trachomatis infection. Scarcity of information in this regard, therefore, necessitates this study.
Materials and Methods
Study Design
This is prospective institutional-based research.
Study Area
This epidemiologic study was carried out among male HIV patients attending HIV clinic at the Nigerian Institute of Medical Research (NIMR), Yaba, Lagos State, Nigeria. NIMR is a government- own research institute which deals with human genomics studies among other things. Yaba is a geographical area located in Lagos state, Nigeria coordinates: 6.5095oN, 3.3711o
Study Duration
The research was carried out between the months of May- July 2022.
Study Population
Adult males aged 18 and older who were HIV and non-HIV positive participated in this cross-sectional institution-based study in Lagos State, Nigeria.
Sample Size Calculation
The sample size for this study was determined using the following formula (Pourhoseingholi, et al. [14]):
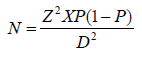
Where:
N= minimum sample size required
Z= confidence interval (1.96)
P= prevalence rate of HIV Chlamydia trachomatis co-infection in a tested population
D= desired level of significance (0.05)
The minimal sample size required was calculated using a 95 percent confidence interval, a P value of 0.019, i.e., a prevalence rate of 10.2 percent for HIV-Chlamydia trachomatis co-infection among male patients from a prior study (Mohammad, et al. [15]), and a margin of error (d) of 0.05. To reduce errors caused by the possibility of non-compliance, 10% of the sample size was added.
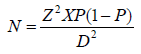
Z =1.96
= 10.2% (Mohammad, et al. [15]).
d = 0.05
N = 1.962x 0.102 (1-0.102)
(0.05)2
N = 3.8416 x 0.102 x 0.898
0.0025
N = 0.3519
0.0025
N = 140.76
N=141
10% of 104: 10/100 x 1 41= 14.1 = 14
Sample size is therefore 141+ 14 = 155
The sample size is 155, based on a prevalence of 10.2% (Mohammad, et al. [15]). The number was scaled up to 159 for homogeneity and precision. Since the study’s goal is to test for Chlamydia trachomatis in two groups of participants: HIV-positive (Test) and non-HIV-positive (Control), A total sample size of 318 was obtained by multiplying the computed sample size by two (2).
Sample Size
A total of 318 samples (blood and urine) were collected 159 HIV-positive and 159 HIV-negative men attending the Nigerian Institute of Medical Research in Yaba, Lagos State.
Ethical Consideration
Ethical clearance was obtained from the Babcock University Health Research Ethics Committee (BUHREC), with ethical registration number: BUHREC 547/22, while the management of the National Institute of Medical Research, Yaba, Lagos State, gave administrative approval before the commencement of the study.
Eligibility of Subjects
Inclusion Criteria
HIV positive and HIV negative males attending the National Institute of Medical Research in Yaba, Lagos State, who are at least eighteen years old and have not received antibiotic therapy in the previous two weeks were randomly selected for the research.
Exclusion Criteria
HIV positive and HIV negative males under the age of 18 who were visiting the Nigerian Institute of Medical Research, Yaba, Lagos State, and who have been on antibiotic therapy in the last two (2) weeks were excluded from the study.
Consent
Each participant was given informed consent. Following a thorough explanation of the study’s objective and nature, as well as the method of sample collection, participants voluntarily completed the permission form in their own handwriting and sign it as proof of their desire to supply samples for the test. They were assured that their information is kept private.
Blood Sample Collection for HIV Detection
Two (2) ml of venous blood samples were collected into plain bottles and allowed to clot to obtain the sera from the patients.
Urine Sample Collection for Chlamydia trachomatis Detection
Five milliliters (5 ml) of first catch morning urine were obtained from the HIV and non-HIV patient and taken to the laboratory for the detection of Chlamydia trachomatis antigen.
Specimen Transportation and Storage
The blood and urine samples were sent to the Department of Medical Laboratory Science, Babcock University, Ilishan-Remo, Ogun State and evaluated within two hours after collection. All samples were transferred to the laboratory as quickly as feasible and processed on the same day they were collected. Each participant’s sample was taken and labeled on the specimen container with their unique identification number. The samples were processed as quickly as possible, they were not stored. But where delay was envisaged, they were kept in the refrigerator at 2-80C.
Laboratory Analyses
HIV Detection
The current National HIV sero-diagnosis methodology was used for HIV detection. This entails using three rapid diagnostic kits in accordance with the manufacturer’s recommendations. Each patient’s serum was examined using Determine (Alere Medical Co. Japan) and Unigold HIV to determine whether they had HIV antibodies (Trinity Biotech Plc Bray, Co. Wicklow, Ireland). The patient is regarded as HIV positive if both kits test positive, and vice versa. A third kit, the Tie Breaker 1/2 Stat Pak (Chembio Diagnostic Systems, New York, USA), is used when test results are ambiguous. One of the first two kits that is compatible with the third kit was used to determine the patient’s HIV serostatus. (Olayanju, et al. [16]; Digban, et al. [17]).
Detection of Chlamydia trachomatis antigen using rapid diagnostic kits
Chlamydia trachomatis antigen in participant’s urine (if present) was identified using DIAGNOSTIC AUTOMATION, INC, Calabasas’ Chlamydia trachomatis rapid test kit. It uses the Chlamydia trachomatis LGV type 2 widely responding antigen. It detects for antigen to Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumonia (TWAR). The test was carried out as directed by the manufacturer.
Principle
Each kit contains a qualitative immunochromatographic lateral flow test in cassette format for detecting C. trachomatis Antigen in urine. The anti-C. trachomatis antibody test is a one-step Chlamydia immunoassay based on the immunochromatographic principle for the rapid, qualitative detection of anti-C. trachomatis Antigen (a clinical specimen is gotten and placed into an extraction tube containing extraction solution in the assay procedure). On the surface of the C. trachomatis test cassette, the letters “T” and “C” stand for “Test Line” and “Control Line,” respectively. Before applying any sample that works successfully, the “Test Line” and “Control Line” in the result are not visible. Procedural control is handled by the “Control Line.” If the test procedure is followed correctly and the control line’s test reagents are operational, the “Control Line” should always appear. If there are enough Antigen against C. trachomatis in the urine sample, a pink “Test Line” will appear in the result window. No colour shows in the “Test Line” if Antigen against C. trachomatis are detected in the sample.
Procedure
• All samples and reagents were warmed to room temperature prior to testing (15-30oC).
• The urine sample were measured in milliliters (mL) and placed in the centrifuge tube. The urine sample was centrifuged at 3000 rpm for 15 minutes.
• The sediments of the urine were obtained, and the supernatant was discarded.
• Reagent B of the Chlamydia kit was added to the sediment and was allowed to stand for 5 minutes after mixing them together.
• After 5 minutes regent A was added to the sediments solution and was allowed to stand for 5 minutes.
• When it was time to start the test, the sealed pouch was opened by tearing along the notch.
• A pipette was used to pour the urine sediment (60l-80l, roughly 2-3 drops) to the sample well on the cassette.
• After 10-15 minutes, the results were read by observing the pink color migrate across the Result Window in the center of the test cassette.
• The result was not read after half an hour.
Interpretation of Results
Positive Result
A positive result is indicated by the appearance of two-color bands both in the test and control line regardless of which band emerges first.
Negative Result
A negative result is indicated by the existence of only one pink color band in the control line
Invalid Result
A complete lack of color in either region or the appearance of only one band on the test region suggests a technique error and/or deterioration of the test reagent.
Statistical Analysis
Microsoft Excel was used to enter the raw data. Statistics software package was used to conduct the data analysis (version 18.0). Significant variations in the prevalence of Chlamydia trachomatis antigen among the study participants was determined using one way analysis of variance (ANOVA). P values of less than 0.05 were deemed significant. Statistical significance is defined as a P-value of less than 0.05. Tables and charts were used to present the results.
Results
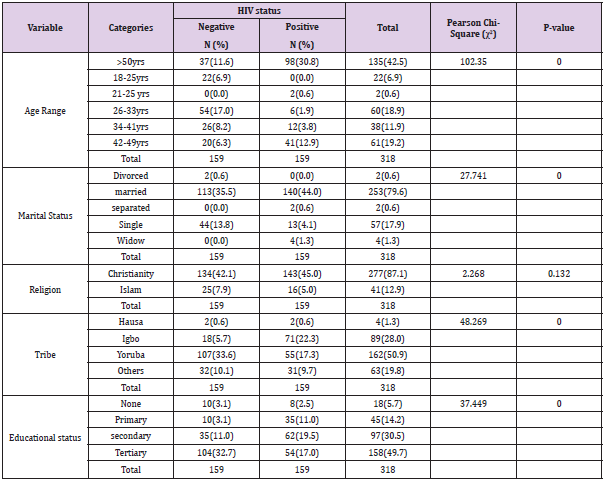
The present study investigated the prevalence of Chlamydia trachomatis antigen among HIV positive and HIV negative adult male patients attending National Institute of Medical Research, Yaba, Lagos State, Nigeria. A total of 318 subjects (159 HIV positive and 159 HIV negative) were enrolled and screened in the study. All study participants underwent a Chlamydia trachomatis test using a One-Step Chlamydia Test kit (GIMA). The sociodemographic distribution of the study individuals is displayed in Table 1 below. Most participants (30.8%) are over the age of 50, whereas only 0.6% of HIV-positive individuals are between the ages of 18 and 25. The same data also reveals that the majority (17.0%) and minority (6.3%) of the HIV-negative participants were, respectively, between the ages of 26-33 and 42-50.
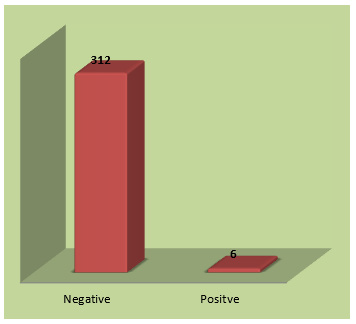
In respect to marital status, majorities (35.5%) were married, and the minorities (0.0) of HIV negative subjects were married and widower. The same figure also reveals that most HIV-positive participants (44.0%) were married and only 0.0% were divorced. Regarding religion, Christians made up the majority (42.1%) of HIV-negative subjects, while Muslims made up the minority (7.9%). The same data also reveals that the majority (45.0%) of HIV-positive individuals were Christians, while the minorities (5.0%) were Muslims. According to the study participants’ educational levels, the bulk of them (32.6%) have completed university education, whereas the minority (3.1%) of HIV-negative individuals has no formal and primary education. Most HIV-positive subjects have secondary education (19.5%), while the minorities (2.5%) have no formal education. The prevalence of Chlamydia trachomatis antigen among the study participants is presented using a bar chart (Figure 1). Overall, regardless of their HIV status, only six (1.9%) out of the 318 of the study participants examined tested positive to Chlamydia trachomatis antigen.
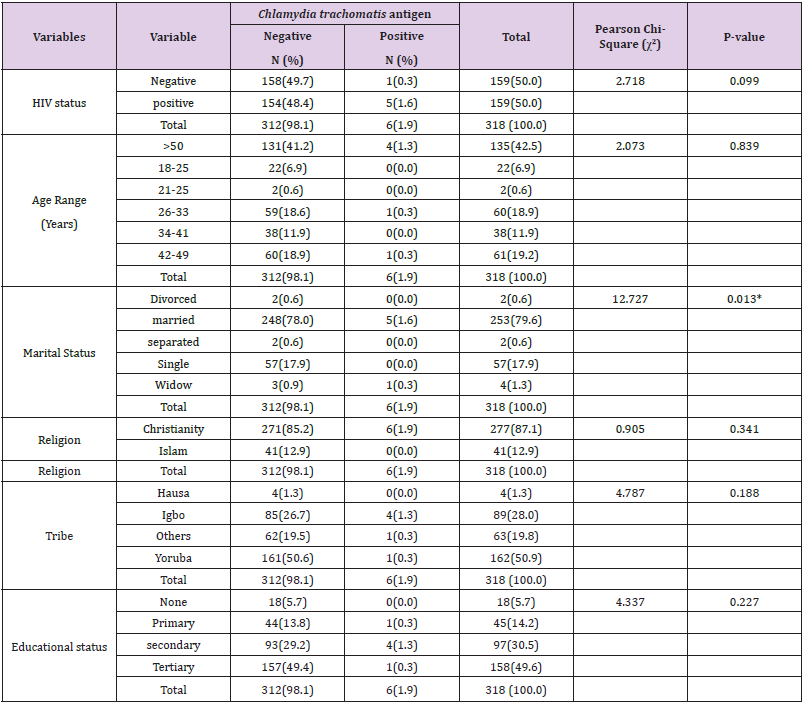
Table 2 shows the prevalence of Chlamydia trachomatis infection based on the socio-demographic details of the study participants. Only one (0.3%) of the 159 study participants who tested negative for HIV had positive Chlamydia trachomatis antigen results, compared to six (1.6%) of the 159 people who tested positive for HIV. Out of 318 study participants, 4 (1.3%) of the participants are 50 years and older, 1 (0.3%) of the participants in the age ranges 26 to 33, and 1 (0.3%) of the participants in the age ranges 42 to 49, tested positive for Chlamydia trachomatis antigen. One widower (0.3%) and five (1.6%) of the study’s participants who were married both tested positive for Chlamydia trachomatis antigen. The married study participants are significantly higher than the widower study participant. Only the Christians with 6(1.9%) out of the study participant tested positive to Chlamydia trachomatis antigen. On the bases tribe 4(1.3%) of Igbos tested positive to Chlamydia trachomatis antigen, 1(0.3%) Yoruba tested positive to Chlamydia trachomatis antigen and then one person which is neither Yoruba, Igbo nor Hausa tested positive to Chlamydia trachomatis antigen. On the bases of educational status, 4 participants (1.3%) with secondary education tested positive to Chlamydia trachomatis antigen, 1 participant (0.3%) with primary education tested positive to Chlamydia trachomatis antigen and then 1 participant (0.3%) with tertiary tested positive to Chlamydia trachomatis antigen.
Table 2: Prevalence of Chlamydia trachomatis infection according to the socio-demographic characteristics of the study participants.
Table 3 lists the signs of Chlamydia trachomatis infection among the research subjects. Out of the 318 study participant some indicated some signs and symptoms, 2(0.6%) of the study participant indicated that have body rashes, 2(0.6%) indicated that they have mild fever, 2(0.6%) indicated to have discharge in penis, 4(1.3%) indicated to have penis sore, 1(0.3%) indicated to have anal sore, 1(0.3%) indicated to have oral sore this study participants all tested positive to Chlamydia trachomatis antigen, none of the study participant which tested positive to Chlamydia trachomatis antigen indicated to having sore throat.
Table 4 shows the risk factors connected to the presence of Chlamydia trachomatis antigen among the study participants. Only 5 (1.6%) of the 6 study participants out of 318 who are positive always take their HAART medication. One person (0.3%) has heard about Chlamydia trachomatis out of the 6-study participant that tested positive to the bacterial. 4(1.3%) out of the 6-study participant which tested positive to Chlamydia trachomatis antigen share sanitary facilities without the other 2(0.6%) that tested positive do not share sanitary facilities. Out of the 6-study participant that tested positive to Chlamydia trachomatis antigen none of them shares underwear with others. Four (1.3%) out the study participant which tested positive to Chlamydia trachomatis antigen change their underwear everyday while 2(0.6%) that tested positive to Chlamydia trachomatis antigen change their underwear every two days. Three, 3(0.9%) out of the 6-study participant that tested positive to Chlamydia trachomatis antigen have history of sexually transmitted infection. Only 1(0.3%) out of the study participant that tested positive to Chlamydia trachomatis antigen engages in unprotected sex. 3(0.9%) out of the study participant that tested positive to Chlamydia trachomatis antigen have 1 sexual partner,1 (0.3%) has 3 sexual partner and 2(0.6%) have no sexual partner. None of the study participant that tested positive to Chlamydia trachomatis antigen have changed sexual partner recently. 4(1.3%) out of the study participant that tested positive to Chlamydia trachomatis antigen go for medical checkup very often, 1(0.3%) go for medical checkup often, 1(0.3%) go for their medical checkup less often.
Discussion
Chlamydia trachomatis (CT) infection is been known as the most prevalent bacterial sexually transmitted disease (STD) worldwide with huge impact on sexual and reproductive health, and because of failure to provide screening and treatment of Chlamydia trachomatis (CT) infection for HIV-infected population the infection begun to spread gradually and become clinically consequential as well as to fuel HIV transmission which makes it common in developed and developing countries (WHO [18]; Sama, et al. [19]).
In this study, we assessed the prevalence of CT antigen and associated risk factors among HIV positive men on HAART in Lagos State, Nigeria using One-Step Chlamydia Test kit (GIMA, Italy). The prevalence rate of 1.6% found in this study is lower than the 5.5% reported by Silva-Santisteban et al. [20] utilizing polymerase chain reaction among HIV-positive men in Central Peru (PCR). A higher prevalence rate reported by Silva-Santisteban et al. [20] may be due to differences in geographical location and methodology used. PCR has been reported to be more sensitive and precise than rapid diagnostic test kits. However, the 1.6% prevalence rate observed in this study is closely like the result of Monteiro, et al. [21] who reported 1.8% among HIV positive women in São Paulo, Brazil.
Comparing the 1.6% identified in this study utilizing the One- Step Chlamydia Test kit (GIMA) to the 45% found by Dangana, et al. [22] using the solid-phase EIA method and an immunocomb Chlamydia IgG kit among HIV men in Abuja, North Central Nigeria, the 1.6% was determined to be extremely low. However, compared to states in other parts of the country, the south-west states have a lower prevalence of HIV, according to the National Agency for the Control of AIDS (NACA, 2000). This helps to explain why there was such a low prevalence of CT in this study.
Furthermore, the 0.3% prevalence rate among HIV negative males in this study was discovered to be much lower than the 62.25% prevalence rate among HIV negative female sex workers reported by Sama, et al. [19] in a study conducted in Cameroon utilizing enzyme-linked immunosorbent test. The study participants’ socioeconomic features, including their gender and occupation, the prevalent environmental conditions in their local environment, and the methods utilized (sensitivity and specificity of the test kits used) could all be contributing factors to this disparity. Most times, especially in females, chlamydia infection has been observed to be asymptomatic. This helps to partially explain the high frequency among female sex workers found by Sama, et al. [19].
Additionally, it was discovered that the 0.3% seen among HIV negative guys in this study was marginally lower than the 2.0% reported by Martin, et al. [23] among University of Dschang, Western Region of Cameroon students using similar rapid diagnostic test kits. With regards to their age, men who are 50 years and above had the highest prevalence rate (1.3%), while the highest prevalence reported by Silva-Santisteban, et al. [20] were within the age range of 18-21 years, owing to their multiple sexual partners. Similarly, highest prevalence rate was reported among 26–45 years (35.6%) and 25-35years (35.29%) in a separate study both carried out in Vhembe District of South Africa by Mafokwane and Samie [24], and Sama, et al. [19], respectively, using real time PCR on urine samples. Likewise, in a study carried out by Okoror, et al. [25], a high prevalence rate of 43.9 % was also reported among women within the age range of 26–30 years, attending gynecological clinics in Southeastern Nigeria.
However, compared to HIV-negative patients, there is evidence that HIV patients are more susceptible to Chlamydia trachomatis infection because of immunosuppression. But, based on the outcome of this study only 1(0.3%) of the participant which tested positive to Chlamydia trachomatis infection engage in unprotected sex and other do use protection which could also made the prevalence of this infection to be less and this could mean that the prevalence of Chlamydia is not always associated with the reported HIV status and also the use of antiretroviral (ARV) may likely appear to reduce the risks and sequelae of Chlamydia infection (Sama, et al. [19]). The co-infection of HIV and Chlamydia trachomatis in the same person is not unusual because both infections are sexually transmitted. Chlamydia trachomatis’ principal symptoms may change because of HIV infection, making it difficult for sufferers to communicate their symptoms.
According to the findings of previous epidemiological studies, Chlamydia trachomatis prevalence among infertile women in Nigeria ranged from 9.6% to 51% (Nwankwo and Magaji, [26]), which is greater than the prevalence found in this study. Though, Dangana, et al. [22] in his study “Prevalence of Chlamydia trachomatis IgG antibodies within the HIV positive women tested when compare to that of the HIV negative women”, no significant increase was observed and buttress his findings by suggesting that it is unlikely that previous exposure to HIV induce a higher prevalence of Chlamydia trachomatis infection, but rather Chlamydia trachomatis infection facilitate the transmission of HIV which is in support with the findings of Mamodou, et al. [27] who also reported that Chlamydia trachomatis infection increases the risk of HIV transmission and acquisition as well further reported that that the invasive intracellular pathogenesis of Chlamydia trachomatis can cause substantial damage to the genital epithelia layer which may facilitate HIV infection (Andersen, et al. [28]) Though, another author reported that sexually transmitted pathogens, including non-ulcerative agent such as Chlamydia trachomatis may serve as biological cofactor for Human Immunodeficiency Virus (HIV) (Ngandino, et al. [29]).
In general, no significant difference was observed between socio-demographical characteristics of the participate and the prevalence of C. trachomatis, except with marital status where significant different was observe regard the present of the infection (X2=12.727, P= 0.013) with high prevalence found among patients who are married. Different from the result of Mafokwane and Samie, [24], whose prevalence rate was found among secondary level of education in Vhembe District of South Africa and different from the result obtain from study conducted in in New Caledonia (Corsenac, et al. [30]). One (1) sex partner only research participant showed a significant difference in risk factors related with Chlamydia trachomatis antigen incidence (X2=10.612, P=0.014), whereas other risk factors remained non-significant.
Also, indications of Chlamydia trachomatis infection among the study participants significant difference was seen from the response given by the participant on the sign such as Body rashes (X2=12.162, P=0.000), Discharge in the penis (X2=18.299, P=0.000), Penis sore (X2=56.332, P=0.000), Anal sore (X2=25.168, P=0.000) and Oral sore (X2= 25.168, P=0.000). The results of this study, however, demonstrate that regardless of HIV status, every infected person with Chlamydia trachomatis infection exhibited symptoms. History of STIs and having unprotected sex do not significantly increase the risk of contracting Chlamydia trachomatis in HIV-positive individuals. Additionally, it was found that HIV subjects with a history of STDs had a 0.391 lower chance of contracting Chlamydia trachomatis infection, whereas those who engaged in unprotected sex had a 2.718 higher risk [31,32].
Conclusion
The outcome of this study shows that CT antigen (1.6%) exists among HIV positive men attending the HIV clinic at National Institute of Medical Research (NIMR), Yaba, Lagos State. Hence, the clarion calls for the facility to include C. trachomatis antigen testing in their routine screening for HIV patients.
Ethical Approval
The Babcock University Health Research Ethics Committee (BUHREC), granted ethical approval for the project with registration number BUHREC 547/22.
Declaration of Interest
The authors report no conflict of interest. The authors alone are responsible for the content of this manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author, [Enitan S. S.], upon reasonable request.
Figure 2: The data that support the findings of this study are available from the corresponding author, [Enitan S. S.], upon reasonable request.
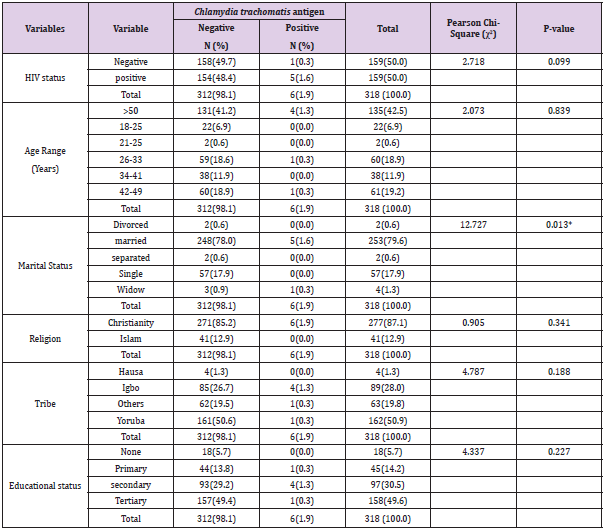
Figure 3: Picture showing a GIMA One Step Rapid Diagnostic Test Cassette negative for Chlamydia trachomatis antigen.
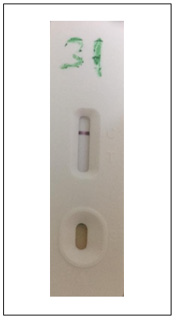
Figure 4: Picture showing a GIMA One Step Rapid Diagnostic Test Cassette positive for Chlamydia trachomatis antigen.
References
- Cheesbrough M (2006) Chlamydiae In: Cheesbrough M (Eds.), District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press, Cape Town, South Africa, pp: 231-234.
- Collee JG, Fraser AG, Marnion BP, Simmons A (1996) Mackie & McCartney Practical Medical Microbiology. 14th pp. 621-630.
- Centre For Disease Control and prevention Miller KE (2006) Diagnosis and treatment of Chlamydia trachomatis American Family Physician. 73(8): 1411-1416.
- Gerbase AC, Zemouri C (2020) Global Epidemiology of Sexually Transmitted Infections in the Twenty-First Century: Beyond the Numbers. Sexually Transmitted Infections 3(12).
- WHO (2011) World Health Organization, Prevalence and incidence of selected sexually transmitted infections Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates.
- Gomes JP, Bruno WJ, Nunes A, Santos N, Florindo C, et al. (2007) Evolution of Chlamydia trachomatis diversity occurs by widespread interstrain recombination involving hotspots. Genome research 17(1): 50-60.
- Wodarz D, Hamer DH (2007) Infection dynamics in HIV – specific CD4 T cell boost benefit the host or the virus? Mathematical Biosciences, 209: 14-29.
- Al-Mutairi N, Joshi A, Nour-Eldin O, Sharma AK, El-Adawy I, et al. (2007) Clinical patterns of sexually transmitted diseases, associated sociodemographic characteristics, and sexual practices in the Farwaniya region of Kuwait. International journal of dermatology 46(6): 594-599.
- Joyce AG, Thyagarajan SP, Rajendran P, R Hari, P Balakrishnan, et al. (2004) Chlamydia trachomatis genital infection in apparently healthy adult population of Tamil Nadu, India: a population-based study. International Journal of STD and AIDS, 15(1): 51-55.
- Joyee AG, Thyagarajan SP, Reddy EV, Venkatesan C, Ganapathy M (2005) Genital chlamydial infection in STD patients: its relation to HIV infection. Indian Journal of Medical Microbiology, 23(1): 37-40.
- Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, et al. (2010) Risk of sequelae after Chlamydia trachomatis genital infection in women. Journal of Infectious Diseases, 201(2): S134-S155.
- WHO (2001) Is Crede's prophylaxis for opthalmia neonatorum still valid? Bulletin of the World Health Organization. 79 (3): 262-266.
- Patel AL, Sachdev D, Nagpal P (2010) Prevalence of Chlamydia infection among women visiting a gynaecology outpatient department: evaluation of an in-house PCR assay for detection of Chlamydia trachomatis. Annals of Clinical Microbiology and Antimicrobials 9:24.
- Pourhoseingholi MA, Vahedi M, Rahimzadeh M (2013) Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 6(1): 14-17.
- Mohammad HA, Akbarn M, Abbas B (2016) Association of Chlamydia trachomatis with infertility and clinical manifestations: a systematic review and meta-analysis of case control studies. Infectious Diseases 48(7): 517-523.
- Olayanju AO, Afolabi T, Ezigbo ED, Enitan SS, Oluwatayo BO (2018) Assessment of antiphospholipid antibodies, CD4 count and some haematological parameters in HIV patients attending a tertiary health institution in south-western Nigeria. International Blood Research and Reviews 8(2).
- Digban TO, Iweriebor BC, Obi LC, Nwodo U, Okoh AI (2019) Molecular Genetics and the Incidence of Transmitted Drug Resistance Among Pre-Treatment HIV-1 Infected Patients in the Eastern Cape, South Africa. Current HIV Research 17(5): 335-342.
- WHO (2015) World Health Organization Fact Sheet, Global Update on the Health Sector Response to HIV, Geneva.
- Sama LF, Noubom M, Nguekam RJ, Dabou S, Tchouangueu TF, et al. (2021) Chlamydia trachomatis IgM and gG antibodies and associated risk factors of among positive and negative HIV women attending consultation at the Mbouo-Banjoun Presbyterian Hospital West Region of Cameroon. RAS Microbiology and Infectious Diseases. 1(2): 2766-838X.
- Silva-Santisteban A, Raymond HF, Salazar X, Villayzan J, Leon S, et al. (2012) Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS and Behavior 16(4): 872-881.
- Monteiro PV, Tancredi TV, da Silva Roberto Jose de Carvalho, Buchalla KZ, Cássia M (2016) Prevalence and factors associated with Chlamydia trachomatis infection among women with HIV in São Paulo. Rev Soc Bras Med Trop. 49(3): 312-318.
- Dangana A, Dantong D, Fredrick CC, Egenti BN (2016) Anti-Chlamydia Trichomatis Igg Positivity Among HIV Positive Women in Abuja, North Central Nigeria. 5(4): 186-192.
- Martin SS, Donfack JH, James-Francis O, Mbongue G, Watcho P, et al. (2015) Prevalence of HIV, HBV and Chlamydia Infections in Cameroonian University Context: Case of the University of Dschang, in the Western Region. International Journal of Health Sciences & Research 5(2): 265-273.
- Mafokwane TM, Samie A (2016) Prevalence of chlamydia among HIV positive and HIV negative patients in the Vhembe District as detected by real time PCR from urine samples, BMC Res Notes. 9(102): 2-10.
- Okoror LE, Agbonlahor DE, Esumeh FI, Umolu PI (2007) Prevalence of chlamydia in patients attending gynecological clinics in southeastern Nigeria. Afri Health Sci 7(1): 18-24.
- Nwankwo EO, Magaji NS (2014) Prevalence of Chlamydia trachomatis infection among patients attending infertility and sexually transmitted diseases clinic (STD) in Kano, Northwestern Nigeria. African Health Sciences. 3(14): 672-678.
- Mamodou S, Laoulkader A, Rabiou S, Aboubacar A, Soumana O, et al. (2006) Prevalence of the HIV infection and five other sexually transmitted infection among sex workers in Niamey, Niger. Bull Soc Pathol Exot. 99: 19-22.
- Andersen B, Olesen F, Moller JK, Ostergaard L (2002) Population-based strategies for outreach screening of urogenital Chlamydia trachomatis infections: A randomized, controlled trial. Journal of Infectious Diseases. 185:
- Ngandino A, Clerc M, Fonkoua MC, Thonno J, Njock F, et al. (2003) Screening of volunteer students in Yazounde (Cameroon, Central Africa) for Chlamydia trachomatis infection and genotyping of isolated Chlamydia trachomatis Journal of Clinical Microbiology. 41: 4404-4407.
- Corsenac P, Noël M, Rouchon B, Hoy D, Roth A (2015) Prevalence and sociodemographic risk factors of chlamydia, gonorrhoea and syphilis: A National multicentre STI survey in New Caledonia, BMJ Open 9(5): 1-9.
- Schust DJ, Ibana JA, Buckner LR, Ficarra M, Sugimoto J, et al. (2012) Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection. Current HIV research 10(3): 218-227.
- Srivastava P, Bhengraj AR, Jha HC, Vardhan H, Jha R, et al. (2012) Differing Effects of Azithromycin and Doxycycline on Cytokines in Cells from Chlamydia trachomatis- Infected Women. DNA and Cell Biology 31(3): 392-401.

 Review Article
Review Article