ABSTRACT
The world’s medical system has undergone a significant upheaval as a result of the catastrophic respiratory Coronavirus Disease (COVID-19), referred to as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). While its effects on respiratory symptoms are widely known, neurological manifestations have remained elusive. SARS-CoV-2 infects host cells by entering via angiotensin-converting enzyme-2 receptors (ACE-2), which are prominently overexpressed on intestinal epithelial cells, endothelial and smooth muscle cells of blood vessels, the heart, lung, renal tubular epithelial cells, and cerebrovascular endothelium. The pathophysiology of SAH in COVID-19 may include several pathways, including coagulopathy, cytokine storm, viral endotheliopathy, hypertension, and immunological regulation. Hemorrhagic cerebrovascular episodes are not uncommon in COVID-19, whether caused by an aneurysmal rupture or spontaneous subarachnoid hemorrhage (SAH). Bleeding into the area around the brain causes a kind of stroke known as subarachnoid hemorrhage (SAH). It is yet unclear what potential processes may be at play when SAH and SARS-CoV-2 are involved. SAH should be investigated in those who have significant neurological symptoms caused by this virus. This review analyses the case of a Caucasian woman who developed SAH in conjunction with COVID-19 infection. As a result, relevant literature and exploration of the putative pathogenetic processes are discussed for the correlation between SARS-Cov-2 and SAH.
Abbreviations: AN: Anorexia Nervosa; BMI: Body Mass Index; INR: International Normalized Ratio; LFTs: Liver Function Tests; SEM: Standard Error of the Mean
Introduction
Presentations of anorexia nervosa (AN) are commonly associated with acute derangement in liver function tests (LFTs). Up to 75% of patients hospitalised with anorexia nervosa demonstrate a relatively mild increase in serum values of hepatocellular enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), typically to less than five times the upper limit of normal, with a much smaller proportion of patients displaying more severe liver injury [1]. The cause of the LFT derangement is linked to nutritional status, with studies demonstrating that the magnitude of rises in serum ALT and AST values is inversely correlated to body mass index (BMI) [1]. Research into the mechanism of liver injury in AN has shown that autophagy plays a significant role [2]. Autophagy is a lysosomal catabolic pathway for degrading cytoplasmic protein and damaged organelles to make them available for recycling. This is a process by which organisms can self-supply nutrients and energy during periods of starvation. Whilst this process is initially protective, when periods of starvation are prolonged, such as in AN, autophagy can lead to an acute liver injury, characterised electrochemically by severe glycogen depletion and a reduced number of mitochondria and endoplasmic reticula [2].
In health, autophagy is regulated by the hormones, insulin and glucagon, and amino acids, principally leucine. Autophagy is prevented by antioxidant defense mechanisms. In the setting of nutritional deficiency and depleted antioxidant defenses leading to oxidative stress, there is increased propensity to liver injury secondary to autophagy [1]. This has led to calls to investigate the possible therapeutic potential of antioxidant therapy in patients with AN [3]. This case series reports the first clinical experiences with antioxidant therapy in patients with severe liver injury related to AN, based on intravenous infusion of the glutathione precursor, N-acetylcysteine, demonstrating that such treatment is associated with significant improvements in parameters of liver injury.
Materials and Methods
We report three severely malnourished patients with AN, managed at a University Teaching Hospital (all female, aged 19 years, 23 years and 27 years, respectively; BMI values 12, 12 and 13, respectively), who presented on a total of seven occasions with nausea, jaundice and severe hepatocellular-type liver injury related to AN (mean serum ALT level 2,974 U/L ± 448 U/L {SEM}, normal <45 U/L; mean serum AST level 3,009 U/L ± 739 U/L {SEM}, normal <45 U/L; mean bilirubin level 62 micromol/L ± 5 micromol/L {SEM}, normal <25 U/L and mean international normalized ratio {INR} 1.8 ± 0.2 {SEM}, normal 0.8-1.1). Alternative causes of liver injury were excluded based on negative laboratory tests for viral (hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis E virus, Epstein-Barr virus, cytomegalovirus, herpes simplex virus, varicella zoster virus, parvovirus), parasitic (Toxoplasmosis gondii), metabolic (haemochromatosis, alpha-1-antitrypsin deficiency, Wilson’s disease) and immune-mediated (autoimmune hepatitis) aetiologias and liver ultrasound, which demonstrated normal liver echogenicity and blood flow without focal liver abnormality or features of biliary obstruction. Serum paracetamol values were undetectable and a urinary drug screen for amphetamines and cocaine was negative on all occasions. There was no history of other potentially hepatotoxic drug exposure.
Following an observation period of 24 hours whilst awaiting the results of the diagnostic laboratory screen for alternative aetiologias of severe liver injury, patients were managed with an intravenous infusion of N-acetylcysteine, 150 mg/kg as a loading dose over 30 minutes and then 150 mg/kg given over 24 hours for a total of 5 days, as used in the management of acute liver failure regardless of aetiology [4]. Laboratory markers (ALT, AST, bilirubin and INR) were measured on days minus 1 and 0 (observation period) and then on days 1, 3, 5 (N-acetylcysteine treatment period) and then on days 7, 14, 21 and 28 (post-treatment observation period).
Results
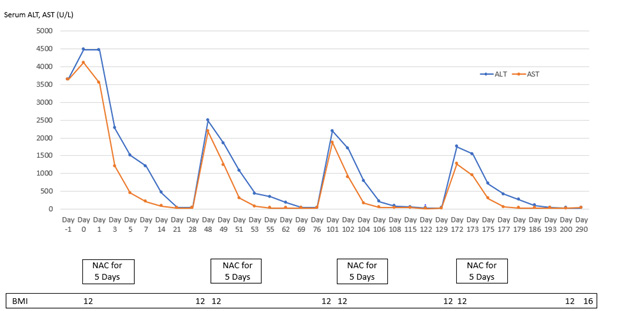
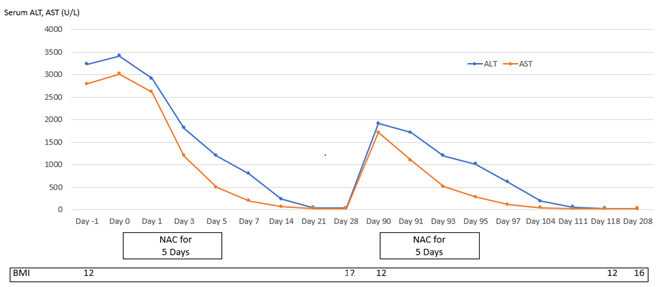
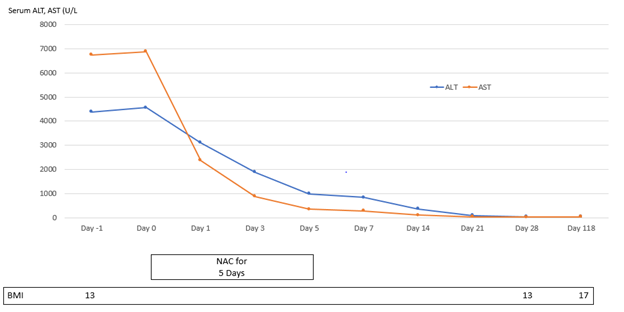
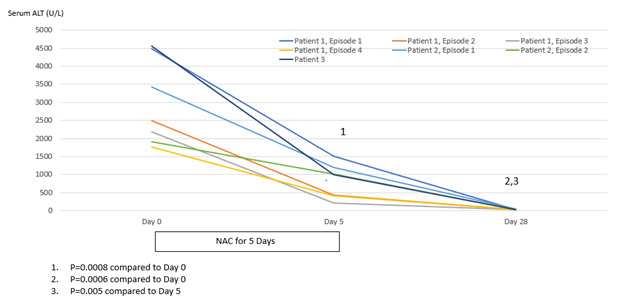
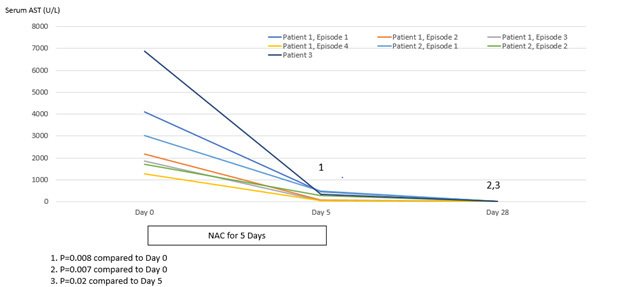
Patient 1 experienced four episodes of severe hepatocellular damage related to AN (Figure 1), whilst Patient 2 experienced two such episodes (Figure 2) and Patient 3 experienced one episode (Figure 3). Serum markers of hepatocellular damage, namely ALT and AST, each increased during an initial 24-hour period of observation prior to the commencement of N-acetylcysteine treatment in all three patients (Figures 1-3). By contrast, commencement of N-acetylcysteine treatment was consistently associated with rapid reductions in serum ALT and AST values, with AST levels typically falling more quickly than ALT levels (Figures 1-3). Statistically significant improvements in ALT and AST levels occurred during the 5-day N-acetylcysteine treatment course, with further statistically significant improvements occurring to day 28, by which time serum ALT and AST values had normalized (Figures 4 & 5). In the two patients with more than one clinical presentation (four subsequent presentations occurring 20 days, 25 days, 43 days and 62 days, respectively, following prior N-acetylcysteine treatment), the magnitude of serum ALT and AST disturbances at subsequent presentations was consistently lower than those found at initial presentation, implying that prior N-acetylcysteine treatment remained partially protective during this time period (Figures 1 & 2).
Figure 1: Markers of hepatic necrosis (serum levels of alanine aminotransferase {ALT} and aspartate aminotransferase {AST}; normal <45 U/L) in relation to intermittent treatment with N-acetylcysteine (NAC) for five days in Patient 1.
Figure 2: Markers of hepatic necrosis (serum levels of alanine aminotransferase {ALT} and aspartate aminotransferase {AST}; normal <45 U/L) in relation to intermittent treatment with N-acetylcysteine (NAC) for five days in Patient 2.
Figure 3: Markers of hepatic necrosis (serum levels of alanine aminotransferase {ALT} and aspartate aminotransferase {AST}; normal <45 U/L) in relation to treatment with N-acetylcysteine (NAC) in Patient 3.
Figure 4: Statistical analysis of serum levels of alanine aminotransferase (ALT) (normal <45 U/L) in relation to treatment with N-acetylcysteine for 5 days and then followed to Day 28.
Figure 5: Statistical analysis of serum levels of aspartate aminotransferase (AST) (normal <45 U/L) in relation to treatment with N-acetylcysteine for 5 days and then followed to Day 28.
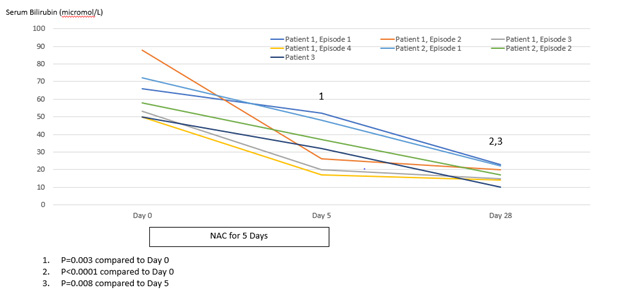
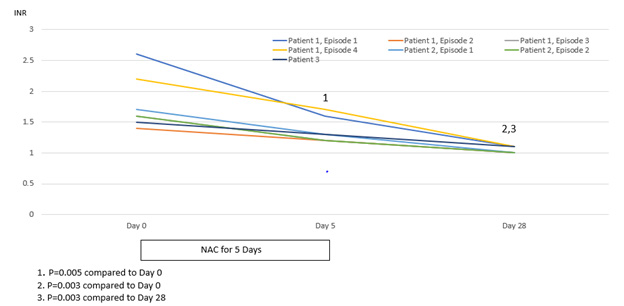
Improvement in hepatocellular injury during N-acetylcysteine treatment was accompanied by statistically significant improvements in the hepatic functional parameters, serum bilirubin and INR, with further statistically significant improvements occurring to day 28, by which time serum levels of these hepatic functional parameters had also normalized (Figures 6 & 7). All three patients remained recalcitrant to re-feeding during the seven presentations, with negligible increases in caloric intake above baseline despite specialist liaison psychiatry input. BMI values remained low throughout the seven periods of N-acetylcysteine treatment in all three patients. An improved nutritional intake leading to improvement in BMI eventually did occur in all three patients and no further instances of severe liver injury developed following this during 90 days of follow-up (Figures 1-3). N-acetylcysteine treatment was well-tolerated by all three patients during all seven treatment exposures, with no instances of any untoward effects.
Figure 6: Statistical analysis of serum levels of bilirubin (normal <25 micromole/L) in relation to treatment with N-acetylcysteine for 5 days and then followed to Day 28.
Figure 7: Statistical analysis of international normalised ratio (INR) values (normal 0.8-1.1) in relation to treatment with N-acetylcysteine for 5 days and then followed to Day 28.
Discussion
This case series demonstrates for the first time that, in patients with AN and severe liver injury, treatment for five days with the antioxidant, N-acetylcysteine, a glutathione precursor, is associated with statistically significant improvements in markers of hepatocellular damage, namely serum ALT and AST levels, as well as statistically significant improvements in the hepatic functional indices, bilirubin and INR. Statistically significant improvements were found to occur during the 5-day treatment period, with further statistically significant improvements occurring to day 28, by which time all indices had normalized. Improvements in liver injury in AN associated with N-acetylcysteine therapy could not be ascribed to natural history, as serum ALT and AST values were found to increase during an initial 24-hour observation period prior to the commencement of antioxidant therapy in all three patients. Neither could any of the improvements in serum ALT, AST, bilirubin and INR values associated with N-acetylcysteine treatment be attributed to an improvement in nutritional status, as caloric intake remained similar to baseline and BMI remained unchanged throughout the treatment and subsequent post-treatment observation periods. An improved nutritional intake leading to improvement in BMI eventually did occur in all three patients and no further instances of liver injury developed following this during a follow-up period of 90 days, in keeping with the notion that liver injury associated with AN is related to a critical reduction in BMI [1].
Importantly, N-acetylcysteine therapy was well-tolerated by our patients, with no instances of side-effects. It has been proposed that treatment with N-acetylcysteine be limited to 5 days in patients with severe liver injury, as its anti-inflammatory effects resulting from inhibition of nuclear factor kappa B expression may predispose to infection in the setting of functional immuneparesis evolving beyond this time [5]. The findings of our study are particularly pertinent, therefore, in that a 5-day treatment duration was found to be sufficient to promote normalization of serum ALT, AST, bilirubin and INR values in patients with severe liver injury associated with AN, while no instances of infection ensued.
Conclusion
We conclude that antioxidant treatment with the glutathione precursor, N-acetylcysteine, is a safe and effective treatment for severe liver injury in patients with AN. Our clinical findings suggest that inadequate antioxidant defense mechanisms likely play a key role in the pathogenesis of severe liver damage associated with AN.
References
- Kheloufi M, Boulanger CM, Durand F, Rautou PE. Liver autophagy in anorexia nervosa and acute liver injury. BioMed Research International 2014. http://dx.doi.org/10.1155/2014/701064.
- Rautou PE, Cazals-Hatem D, Moreau R, Francoz C, Feldmann G, et al. (2008) Acute liver cell damage in patients with anorexia nervosa: A possible role of starvation-induced hepatocyte autophagy. Gastroenterology 135(3): 840-848.
- Solmi M, Veronese N, Manzato E, Sergi G, Favaro A, et al. (2015). Oxidative stress and antioxidant levels in patients with anorexia nervosa: a systematic review and exploratory meta-analysis. International Journal of Eating Disorders 48: 826-841.
- Wendon JA, Ellis AJ (1997) Circulatory derangements, monitoring and management: heart, kidney and brain. In: Lee WM, Williams R (Eds.)., Acute Liver Failure; Cambridge University Press, Cambridge, United Kingdom, pp. 132-143.
- (2017) European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. Journal of Hepatology 66: 1047-1081.

 Case Report
Case Report